2742
Estimation of Fatty Acid Composition in Mammary Adipose Tissue Using Unsupervised Approach of Deep Learning1Radiology, Weill Cornell Medical College, New York, NY, United States, 2Weill Cornell Medical College, New York, NY, United States, 3Weill Cornell Medical college, New York, NY, United States, 4Weill Cornell Medical College, New york, NY, United States, 5MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 6New York University School of Medicine, New York, NY, United States
Synopsis
Keywords: Breast, Cancer
The purpose of this study is to develop a non-invasive imaging method to measure the fatty acid composition (FAC) of mammary adipose tissue (MAT) and to investigate its role in breast cancer. A novel unsupervised deep learning approach has been developed using the MRI signal equation of fat peaks in the loss function to generate the FAC maps without using any training data. It takes less computational efforts than conventional voxel-wise analysis techniques. The repeatability and reproducibility of the proposed method have been examined on six subjects, which showed no statistically significant difference between repeated analyses and scans.Introduction
Previous studies have shown that mammary adipose tissue plays an important role in breast cancer development.1,2 Furthermore, it has also been observed that Fatty Acid Composition (FAC) is significantly different between postmenopausal women with and without breast cancer.3,4 While MRI can be used to measure FAC of mammary adipose tissue (MAT) non-invasively with a high spatial resolution,3,4,5 it typically requires a voxel-wise linear/non-linear model fitting process which is slow and a significant hinderance for clinical translation and application of this method with a larger cohort. Hence, the purpose of this study is to develop a Deep Neural Network (DNN) technique for rapid measurement of FAC in mammary adipose tissues with a high spatial resolution.Method
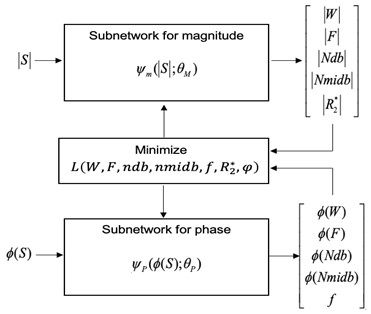
We have developed a physics-informed DNN using an unsupervised approach with no training data. The proposed method is an extension of the work by Jafari et al6 in which the water and fat images were separated using an unsupervised DNN with no training data. We extend the approach for FAC estimation using the 10-peak fat model7,8 while using bipolar gradient echo data. Data acquisition was conducted with an modified GRE sequence for this research with 16 bipolar echoes (FA= 10°, TR = 471 ms, inter-echo spacing = 1.23 ms, receiver bandwidth = 1950 Hz/pixel, in-plane matrix size = 256 x 256, 30 slices, and total acquisition time = 1.2 min). The DNN consisted of two 2D U-Nets with an identical structure for encoding and decoding paths (Fig.1). Input for one U-Net is the magnitude part of the complex data while in other 2D U-net, the phase part of the data is used. FAC parameters, including the number of double bonds (ndb), number of methylene-interrupted double bonds (nmidb) and chain length(cl) while chain length is kept as function of ndb per triglyceride molecule (cl=16.8+0.25 ndb), were estimated using the DNN with the following loss function:$$$L(W,F,ndb,nmidb,f,R_2^*,φ)=\sum_{j=1}^N\parallel S_j-(W+Fk\sum_{p=1}^{10}ρ_p e^{-i2πδ_p t_j}) e^{-R_2^* t_j} e^{-i2πft_j } e^{i(-1)^j φ}\parallel_2^2+λ\parallel TV(f)\parallel_{1}$$$
where is the j-th echo signal, W is water content, F for fat content, $$$K=1⁄∑_{p=1}^{10}ρ_p$$$ ,f for frequency offset, $$$φ$$$ for the phase discrepancy between even and odd, $$$ρ_{p} (p = 1, …, 10)$$$ is the amplitude of ten distinct fat peaks that are chemically shifted by δp with respect to water and TV for total variation. The estimated ndb, nmidb and cl are converted to saturated fatty acid (SFA) and monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acid
Results & Discussion
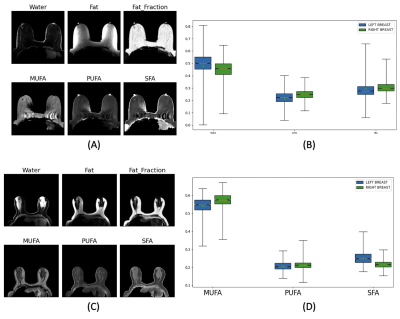
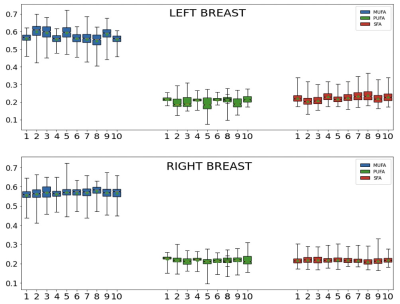
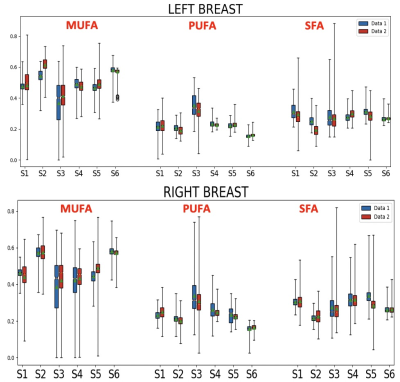
The proposed DNN was successfully implemented. Figures 2 show two representative cases. Subject-1 has noticeable motion and susceptibility artifacts in the raw images as well as FAC maps. However, the effect appears negligible in the FAC maps outside the artifacts. Subject-2 is a representative case without any noticeable artifact in which the proposed DNN generated mostly even FAC maps without any artifact. The FAC values are within the range of FAC values reported for mammary adipose tissue. The repeatability of the DNN was assessed by running the analysis 10 times using a same data set as shown in Figure 3, suggesting the variability among repeated analyses is in the order of or less than the difference between the left and right breasts in Figure 2. The reproducibility of the proposed method was assessed by using the test-retest scans conducted within a same imaging session per subject (Figure 4). There is no statistically significant difference in the FAC values between the two data sets of the subjects. The proposed DNN method has been successfully developed to generate the MUFA, PUFA and SFA maps of the breast adipose tissue that are within the expected range of values in the literature. We are currently extending this study to measure 3D FAC maps of the mammary adipose tissue in postmenopausal women with and without breast cancer.Conclusion
Our preliminary result suggests that the physics- informed DNN developed in this study can be used to estimate MUFA, PUFA & SFA images without prior training of the DNN. This approach will be used to assess the role of FAC in breast cancer development and treatment response.Acknowledgements
NIH R01CA219964, R01CA160620 and UH3CA228699References
1) Yamaguchi, Hiroshi Ohtani, Kazukuni Nakamuraet al, Prognostic Impact of Marginal Adipose Tissue Invasion in Ductal Carcinoma of the Breast,American J of Clinical pathology 2008;130(3):382-8.
2) Zhu, Harvey S, et al, Invasive Breast Cancer Preferably and Predominantly Occurs at the Interface between Fibroglandular and Adipose Tissue, Clin Breast Cancer. 2017;17(1): e11-e18.
3) Freed, Pippa Storey, A.A Lewin, S. Gene Kim et al. Evaluation of Breast Lipid Composition in Patients with Benign Tissue and Cancer by Using Multiple Gradient-Echo MR Imaging Radiology 2016; 281(1):43-53.
4) Lewin, Pippa Storey, Melanie, Linda Moy, S. Gene Kim et al. Fatty acid composition in mammary adipose tissue measured by Gradientecho Spectroscopic MRI and its association with breast cancers, European Journal of Radiology 2019;116: 205-211.
5) Baboli ,Pippa Storey, Melanie, Linda Moy, S. Gene Kim et al, Bilateral gradient-echo spectroscopic imaging with correction of frequency variations for measurement of fatty acid composition in mammary adipose tissue, Magn Reson Med. 2021;00:1–13.
6) Jafari , J Zhang, Yi Wang et al, Deep neural network for water/fat separation: Supervised training, unsupervised training, and no training, Magn Reson Med. 2021;85:2263–2277.
7) Bydder, Olivier Girard, G. Hamilton, Mapping the double bonds in triglycerides, Magn Reson Med 2011;29: 1041–1046.
8) Berglund, Ahlström H, Kullberg J, Model-Based Mapping of Fat Unsaturation and Chain Length by Chemical Shift Imaging—Phantom Validation and In Vivo Feasibility, Magn Reson Med 2012;68:1815–1827.
Figures