2737
Imaging Microstructural Parameters of Breast Tumor in Patient Using Temporal Diffusion Spectroscopy
Shuyi Peng1, Peng Sun2, Zhigang Wu2, and Fan Yang1
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Philips Healthcare, Beijing, China
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Philips Healthcare, Beijing, China
Synopsis
Keywords: Breast, Diffusion/other diffusion imaging techniques, OGSE; IMPULSED; Breast tumor
This study aimed to investigate the feasibility of temporal diffusion spectroscopy (the IMPULSED technique) for breast imaging. The result shows that the diffusion MRI–based microstructural mapping can evaluate the cell size in breast tumors and may demonstrate pathologic findings promise for characterizing breast cancer.Purpose
Diffusion-weighted imaging (DWI) has been investigated as a means of displaying microstructure of breast lesions and is used to detect changes in the apparent diffusion coefficient (ADC) to reflect the cellular nature of the tumor. However, ADC only reflects the overall measurement of water diffusion rate and is determined by a variety of microstructure characteristics, such as intracellular and extracellular space, cell size, permeability, and inherent diffusion rate[1, 2]. Therefore, more accurate and specific imaging markers are needed to display specific tumor microstructures to accurately describe the pathological characteristics of breast tumors. Recent studies have suggested that the imaging microstructural parameters using limited spectrally edited diffusion (IMPULSED) approach can be used to analyze diffusion-weighted MRI signals and derive specific microstructural parameters to characterize the average cell size in solid tumors[1, 3, 4]. Thus, this work aimed to investigate the feasibility of the IMPULSED technique on microstructural mapping for characterizing cellular properties of breast cancer.Methods
The study was approved by the institutional ethics committee. From October 2022 to November 2022, 9 patients suspected of breast cancer underwent breast MRI examinations on a 3T MRI scanner with a dedicated 16-channel phased-array breast coil (Ingenia CX, Philips Healthcare). All the patients with malignant breast lesions were histopathologically confirmed by biopsy. Patients underwent bilateral breast MRI using a combination of oscillating gradient spin-echo (OGSE) and pulsed gradient spin-echo sequences to capture the diffusion images.The details of acquisition sequence parameters were: TR/TE = 4000/105ms; FOV = 192×192 mm; reconstructed in-plane resolution = 2×2 mm; slice thickness = 5 mm. The OGSE data were acquired at oscillating frequencies of 25 Hz (effective diffusion time = 10 ms, one cycle, b = 0, 250, 500,750, and 1000 s/mm2) and 50 Hz (effective diffusion time = 5 ms, two cycles, b = 0, 100, 200and 250 s/mm2), and pulsed gradient spin-echo at effective diffusion time of 78.4 ms (b = 0, 250, 500, 750, 1000, 1400 and 1800s/mm2). The data are fitted using in-house written MatLab scripts consulting the open-source code released by the author of IMPULSED (https://github.com/jzxu0622/mati.git), after denoised by using MRtrix3 (https://www.mrtrix.org/) package and motion-corrected by using the FSL package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/).Results
Figure 1 shows IMPULSED signals from a tumor ROI and the IMPULSED fits (solid lines) of a representative human breast tumor. Figure 2 shows the histogram of the fitted pulse index of the tumor, it shows that the peak value of d is between 14 μm and 16 μm, and the peak value of νin is in the range of 20% to 30%. The fitted overall mean cell size was d = 14.65 ± 2.14 μm, νin =0.31 ± 0.12, and Dex = 1.83 ± 0.69 μm2 /ms, which is consistent with the previous report that the average cell size of 11-18μm for breast tumors[3]. Figure 3 shows the IMPULSED-derived maps of mean cell size d, intracellular volume fraction νin, and extracellular diffusion coefficient Dex, the parametric maps within the tumor are uneven, indicating the heterogeneity within the tumor.Conclusion
The results in this study suggest that the IMPULSED approach can assess cell size in breast tumors, which may provide complementary information on tumor grading and prediction of early response to neoadjuvant therapy.Acknowledgements
No acknowledgement found.References
- Jiang, X.; Xu, J.; Gore, J.C. Mapping hepatocyte size in vivo using temporal diffusion spectroscopy MRI. MAGN RESON MED 2020, 84, 2671-2683.
- Jiang, X.; Li, H.; Xie, J.; McKinley, E.T.; Zhao, P.; Gore, J.C.; Xu, J. In vivo imaging of cancer cell size and cellularity using temporal diffusion spectroscopy. MAGN RESON MED 2017, 78, 156-164.
- Xu, J.; Jiang, X.; Li, H.; Arlinghaus, L.R.; McKinley, E.T.; Devan, S.P.; Hardy, B.M.; Xie, J.; Kang, H.; Chakravarthy, A.B.; Gore, J.C. Magnetic resonance imaging of mean cell size in human breast tumors. MAGN RESON MED 2020, 83, 2002-2014.
- Wu, D.; Jiang, K.; Li, H.; Zhang, Z.; Ba, R.; Zhang, Y.; Hsu, Y.C.; Sun, Y.; Zhang, Y.D. Time-Dependent Diffusion MRI for Quantitative Microstructural Mapping of Prostate Cancer. RADIOLOGY 2022, 303, 578-587.
Figures
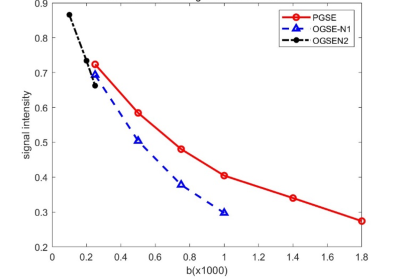
Figure 1. The ROI-based diffusion-weighted signal of the breast tumor. Markers are mean signals, and the solid lines are fitted results.
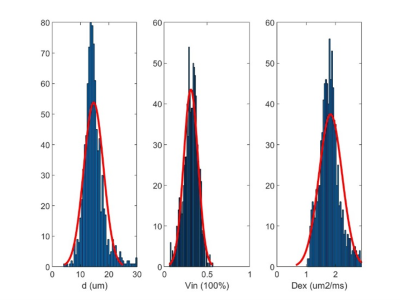
Figure 2. The histogram of fitted IMPULSED metrics of breast tumor.
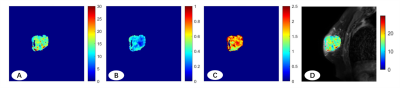
Figure 3. (A, B, C) IMPULSED-derived maps of mean cell size d (A), intracellular volume fraction nin (B), and extracellular diffusion coefficient Dex (C); (D) IMPULSED-derived maps of mean cell size d overlaid on the corresponding diffusion-weighted image.
DOI: https://doi.org/10.58530/2023/2737