2736
Ultrashort Echo Time (UTE) MRI in the Detection and Classification of Microcalcification in Breast Cancer1Aberdeen Biomedical Imaging Centre, Institute of Medical Sciences, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, United Kingdom, 2Department of Pathology, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 4Newcastle Magnetic Resonance Centre, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle, United Kingdom
Synopsis
Keywords: Breast, Cancer
Micro-calcification is a central feature of breast cancer, and clinically revealed on mammography. Mammography, a two dimensional imaging approach, is inadequate to provide refined classification. Conventional MRI cannot capture the rapid signal decay from micro-calcification, with Ultra Short Echo Time (UTE) developed to image short T2* species. UTE demands precise control of the hardware to minimise the time delay between radiofrequency transmission and image acquisition, while substantial effort is required to optimise image quality and contrast. We set out to examine the degree of calcification in breast tumour specimens freshly excised from patients using UTE, with comparison against histopathological findings.
Introduction
Breast cancer is the most prevalent cancer affecting women, while the deposition of calcium in solid form, known as calcification, is a central feature1. The detection and classification of calcification in breast cancer holds significant prognostic value in treatment planning2. Mammography, as the only current clinical radiological approach sensitive to the presence of calcification, cannot reveal the precise classification of calcification3. Ultrashort Echo Time (UTE) imaging, a novel radiological method, addresses the limitation of conventional MRI to primarily soft tissue application by capturing rapid signal decay in solid state matters such as calcification. However, UTE demands non-cartesian scanning trajectory and high-performance scanner hardware to minimise the time lag between excitation and signal detection, and only became adequately robust and available on clinical scanners recently4. We therefore set out to examine the feasibility of UTE imaging in identifying histological characteristics of microcalcifications in freshly excised breast tumours.Methods
Twenty breast tumour specimens were removed from female patients undergoing wide local excision, mean (range) age of 57 (35 – 78) years, with invasive ductal carcinoma: 10 with grade II and 10 with grade III. The fresh specimens were immediately placed in 10% buffered solution of formalin at surgery. UTE imaging was performed to derive degree of calcification, and standard pathological analysis was conducted to determine the tumour grade, calcification status, Nottingham Prognostic Index (NPI) and proliferative activity marker Ki-67 to assess differences arising from clinical features. The study design is shown in figure 1. The study was approved by the North West-Greater Manchester East Research Ethics Committee (REC reference number: 16/NW/0221), and signed written informed consent was obtained from all patients prior to entry into the study.Image Acquisition: The specimens were scanned on a 3T whole-body MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using a 32-channel receiver coil for high sensitivity detection and a body coil for uniform transmission. For tumour localisation, anatomical images were acquired using standard T1-weighted and T2-weighted sequences with conventional diffusion weighted images (2 b-values of 0 and 800 sec/mm2). The UTE images were acquired with a 3D-radial dual echo UTE sequence, with echo times (TE) of 0.17 ms and 4.60 ms, repetition time (TR) of 8.5 ms, FOV of 141 × 141 mm2, voxel size of 2.2 × 2.2 × 2.2 mm3.
Image Analysis: The whole tumour was manually delineated across the tumour volume with the aid of T1 and T2 anatomical images in MATLAB (Mathworks Inc., Natick, USA) (figure 2). Subsequently, the signal intensity of the two echoes were derived as the mean of the image intensity within the whole tumour for each echo. The degree of calcification was computed as the signal difference between the two echoes normalised by the long echo. The tumour size was quantified as the maximum diameter and the average area (spatial extent) of the whole tumour.
Statistical Analysis: All statistical analysis was carried out using SPSS software (Release 27.0, SPSS Inc, IL, USA). The degree of calcification from UTE between histological calcification status (malignant, benign or no calcification) was compared using one-way ANOVA. A Tukey's HSD post hoc tests were conducted to indicate the significance of differences among the three calcification groups. Spearman’s correlation tests were performed between degree of calcification against NPI and Ki-67 scores. An independent t-test was conducted to compare the differences in the degree of calcification between tumour grades II and III.
Results
There was a significant difference (P = 0.012) in the degree of calcification between specimens with malignant calcification (1.054 ± 0.132) and specimens with no calcification (0.842 ± 0.091) (figure 3). There was no significant difference (P = 0.355) in the degree of calcification between specimens with malignant calcification (1.054 ± 0.132) and benign calcification (0.951 ± 0.158). There was no significant correlation between the degree of calcification against NPI and Ki-67 scores (figure 4). A detailed summary of the results is shown in Table 1.Discussion
UTE enhances the detection of short T2* species and may have potential in the detection of solid state signal from calcifications in breast tumours. We examined the degree of calcification in tumour specimens through the separation of quantitative solid state signal in the short first echo from the soft tissue signal in the second long second echo, using dual echo UTE approach Although UTE showed a potential in sensitivity to calcified tumours, further work is required for non-invasive differentiation of calcification classes. Malignant calcifications have a crystalline structure as a result of the formation of hydroxyapatite crystals, while benign calcifications, generally composed of calcium oxalate crystals, may form amalgamate with hydroxyapatite crystals, introducing challenges in the differentiation of solid state signals5. No correlation between histologic calcification classes and tumour grade and size was found, although both are reported to have an association with calcified tumours6.Conclusion
Tumours with malignant calcification may be differentiated from tumours without calcification using UTE imaging, while benign calcification did not show a significant difference.Acknowledgements
The author would like to thank Dr Matthew Clemence (Philips Healthcare Clinical Science, UK) for clinical scientist support, and Ms Bolanle Brikinns and Ms Dawn Younie for patient recruitment and logistic support.References
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019 Nov;69(6):438-451.
Figures
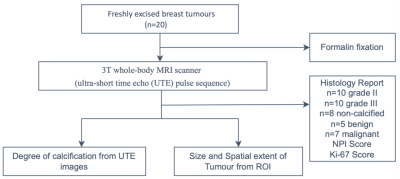
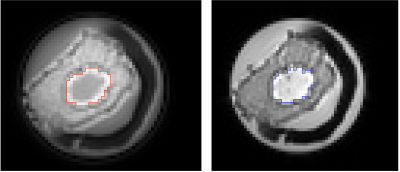
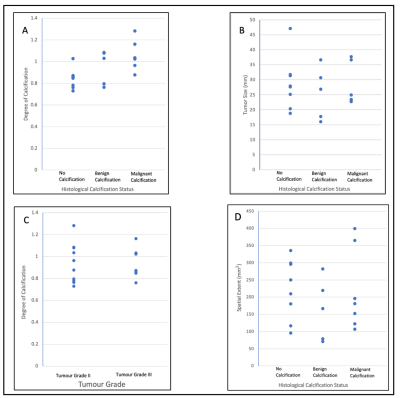
Figure 3: Graphical representation of UTE image analysis in relation with histology data. (A) Degree of calcification based on the three calcification groups, (B) Tumour size based on the three calcification groups, (C) Degree of calcification based on the tumour Grade II or III, (D) Spatial extent based on the three calcification groups
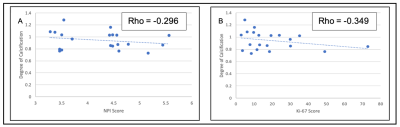
Figure 4: Association between degree of calcification against NPI and Ki-67 scores. (A) Correlation plot between the degree of calcification and the NPI score, (B) Correlation plot between the degree of calcification and the Ki-67 score
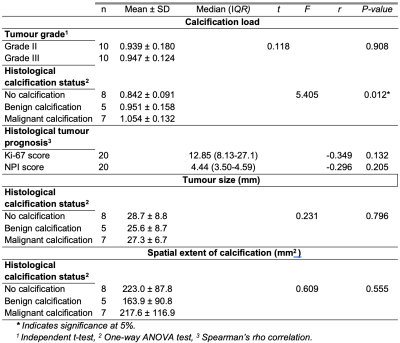
Table 1: A summary of the results and statistical tests