2729
Diagnostic performance of DCE-MRI for the axillary sentinel lymph nodes status in breast cancer patients1Department of Radiology,The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China, 2The Second Affiliated Hospital of Chengdu Medical College, Nuclear Corportation 416 Hosptital, chengdu, China, 3GE Healthcare, MR Research, Beijin, China
Synopsis
Keywords: Breast, Cancer, contrast-enhanced (DCE) MRI, sentinel lymph nodes
Sentinel lymph nodes(SLN) status is one of the most important indicators affecting breast cancer patient treatment and prognostic. Thus, a non-invasive diagnostic method capable of accurately predicting of the SLN status is of great value in selecting breast cancer patients who would benefit from therapy. In this study, we investigate the diagnostic performance of different SLN status [pN0, pN0 (i+), pN1mi, and pN1] using dynamic contrast-enhanced (DCE) MRI.We found that (1) Krans, Kep, and Ve are significantly different in different SLN status, (2) and the Ktrans is the best single parameter for the detection of SLN status.Introduction
Breast cancer is one of the most common malignant tumors in women worldwide. Sentinel node biopsy for breast cancer patients is a targeted assessment of lymph nodes has been applied as an alternative to axillary lymph node dissection since the 1990s [1-2]. In the past, the detection rate of micro-metastases of sentinel node is lower due mainly to the limitations of the detection approaches. Since the development of novel tracers and pathological and histological techniques, the most important nodes (the SLNs) undergo a more thorough and standardized histopathologic examination, which improved detection rate of isolated tumor cells (ITCs) and micrometastases in the SLNs [3-4]. Recently years, quantitative parameters derived from various MRI sequences imaging data have proven to be useful for the diagnosis metastatic and nonmetastatic SLNs in patients with breast cancer [5-8]. However, the value of quantitative parameters from MRI in the diagnosis of different sentinel lymph node status [pN0, pN0(i+), pN1mi, and pN1] in patients with breast cancer remains elusive. In this study, we investigate the diagnostic performance of quantitative parameters derived from DCE-MRI for different axillary sentinel lymph node status in breast cancer patients.Methods
This retrospective study was performed with the clinicopathologic and radiologic image data of breast cancer lumps patients who underwent SLN biopsy and axillary lymph node dissection between July 01, 2019, and December 16, 2021 at department of breast surgery in the First Affiliated Hospital of University of Science and Technology of China. All examinations were carried on a 3.0T MRI scanner (Discovery, MR750W GE Healthcare, Milwaukee, USA). According to lymph node staging after surgery, patients were divided in to four groups: node negative (pN0), isolated tumor cells [≤0.2 mm, pN0 (i+)], micrometastases (>0.2–2 mm, pN1mi), macrometastases (>2 mm, pN1). DCE-MRI-derived kinetic parameters (time intensity curves shapes, maximum slope, Ktrans, Ve, Kep) were assessed for each lesion for breast cancer lumps (Figure 1). Quantitative DCE-MRI kinetic parameters and morphologic parameters were compared between the metastatic tumor in axillary lymph nodes. The quantitative and semi-quantitative parameters were fitted to univariable and ordered logistic regression models. the diagnostic performance of morphologic and quantitative parameters was quantified by receiver operating characteristic curve and compared by using the McNemar test.Results
Result: 58 participants (all of female, with ages range from 22 to 80, a mean value is 50.28 ± 11.09) are eligible in this study. Quantitative DCE-MRI parameters including maxslope of the time intensity curves, Krans, Kep, and Ve were statistically significantly different across every sentinel lymph node status (Figure 2-3). Univariable and ordered logistic regression analyses demonstrated that Ktrans is the best single parameter for the detection of sentinel lymph node status. The area under the receiving operating characteristic curve (AUC) of the Ktrans for detecting metastatic and nonmetastatic SLNs is 0.942 (95% CI 0.887–0.997, P < 0.001). The Ktrans has an AUC of 0.937 (95% CI 0.874–1.000, P < 0.001) for distinguishing SLNs with a pathological grade range of pN0 - pN1mi from those macrometastases for distinguishing SLNs. Ktrans has an AUC of 0.928 (95% CI 0.855–1.000, P < 0.001) for distinguishing a pathological grade range of pN0 - pN0(i+) from the grade range of pN1mi - macrometastases (Figure 4).Discussion
Morphological criteria and quantitative parameters such as T1, T2, and ADC value were suggested to be insufficient for the differentiation of each sentinel lymph nodes status [9-10]. Another study found that venous phase lHu in dual-energy CT is the best single parameter for the preoperative identification of sentinel lymph nodes metastases in participants with breast cancer [11].Choi EJ et al used DCE-MRI and DWI (diffusion-weighted imaging) to evaluate the diagnostic value of metastatic extremely non-metastatic lymph nodes, they found that internal enhancement on DCE-MRI and peritumoral-tumoral ADC ratio on DWI is independently associated risk factor with SLN metastasis, but quantitative parameters of DCE-MRI (E1a, Epeaka, SERa, TTPa) are not an independent risk factor [12]. However, our study showed that quantitative DCE-MRI parameter Ktrans has better diagnostic potential (for both specificity and accuracy) for the preoperative identification of axillary SLN status in participants with breast cancer than other morphologic parameters such as maxslpoe, curve type, Ve and Kep. One plausible reason is that the tissue permeability information is faithfully represented in the quantitative parameter of Ktrans.Conclusion: In this study, fifty-eight patients were divided into four groups according to the sentinel lymph node histopathologic stages: node negative (17 patients, 29.3%), isolated tumor cells (10 patients, 17.2%), micrometastases (11 patients, 19.0%) and macrometastases (21 patients, 34.5%). Ktrans is the best single parameter for the detection of sentinel lymph node status. DCE-MRI parameter Ktrans is useful for distinguish the metastatic tumor status in axillary sentinel lymph nodes in breast cancer patients.Conclusion
In this study, fifty-eight patients were divided into four groups according to the sentinel lymph node histopathologic stages: node negative (17 patients, 29.3%), isolated tumor cells (10 patients, 17.2%), micrometastases (11 patients, 19.0%) and macrometastases (21 patients, 34.5%). Ktrans is the best single parameter for the detection of sentinel lymph node status. DCE-MRI parameter Ktrans is useful for distinguish the metastatic tumor status in axillary sentinel lymph nodes in breast cancer patients.Acknowledgements
Acknowledgments: We would a like to thank Miaoqi Zhang and Zhang Yong for conducting the data analysis.References
[1] Tavora B, Mederer T, Wessel KJ, Ruffing S,et al. Tumoural activation of TLR3-SLIT2 axis in endothelium drives metastasis. Nature. 2020;586(7828):299-304.
[2] Andersson Y, Bergkvist L, Frisell J,et al. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat. 2018 ;171(2):359-369.
[3] Bañuelos-Andrío L, Rodríguez-Caravaca G, Argüelles-Pintos M,et al. Validez diagnóstica del análisis intraoperatorio mediante sección en congelación del ganglio centinela en el manejo quirúrgico del cáncer de mama [Diagnostic validity of the intraoperative analysis in frozen section of the sentinel lymph node in the surgical management of breast cancer]. Rev Esp Med Nucl Imagen Mol. 2014;33(4):193-8.
[4] Dixon JM, Grewar J, Twelves D,et al. Factors affecting the number of sentinel lymph nodes removed in patients having surgery for breast cancer. Breast Cancer Res Treat. 2020;184(2):335-343.
[5] Onishi N, Sadinski M, Hughes MC, et al. Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res. 2020;22(1):58.
[6] Zhang M, Horvat JV, Bernard-Davila B,et al. Multiparametric MRI model with dynamic. contrast-enhanced and diffusion-weighted imaging enables breast cancer diagnosis with high accuracy. J Magn Reson Imaging. 2019;49(3):864-874.
[7] Graña-López L, Pérez-Ramos T, Maciñeira FA,et al. Predicting axillary response to neoadjuvant chemotherapy: the role of diffusion weighted imaging. Br J Radiol. 2022;95(1130):20210511.
[8] Baran MT, Gundogdu H, Demiral G,et al. PET-CT and MR Imaging in the Management of Axillary Nodes in Early Stage Breast Cancer. J Coll Physicians Surg Pak. 2020;30(9):946-950.
[9] Korteweg MA, Zwanenburg JJ, Hoogduin JM, et al. Dissected sentinel lymph nodes of breast cancer patients: characterization with high-spatial-resolution 7-T MR imaging. Radiology 2011;261(1):127–135.
[10] Rahbar H, Conlin JL, Parsian S, et al. Suspicious axillary lymph nodes identified on clinical breast MRI in patients newly diagnosed with breast cancer: can quan- titative features improve discrimination of malignant from benign? Acad Radiol 2015;22(4):430–438.
[11] Zhang X, Zheng C, Yang Z,et al. Axillary Sentinel Lymph Nodes in Breast Cancer: Quantitative Evaluation at Dual-Energy CT. Radiology. 2018;289(2):337-346.
[12] Choi EJ, Youk JH, Choi H,et al. Dynamic contrast-enhanced and diffusion-weighted MRI of invasive breast cancer for the prediction of sentinel lymph node status. J Magn Reson Imaging. 2020;51(2):615-626.
Figures
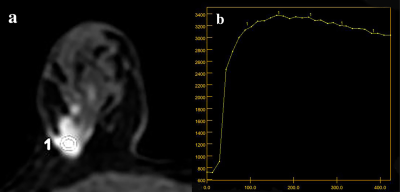
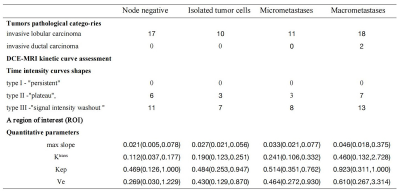
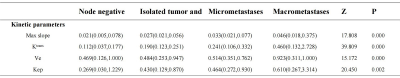
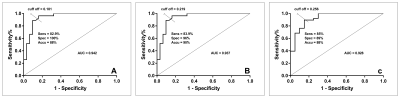
FIGURE 4: Accu = accuracy, AUC = area under the curve, sens = sensitivity, spec = specificity. The area under the receiving operating characteristic curve (AUC) of the Ktrans for detecting metastatic and nonmetastatic SLNs is shown as Figure(4a). Figure(4b) for distinguishing SLNs with a pathological grade range of pN0 - pN1mi from those macrometastases for distinguishing SLNs. Figure(4c) for distinguishing a pathological grade range of pN0 - pN0(i+) from the grade range of pN1mi - macrometastases.