2722
The ALFF alterations in maintenance hemodialysis patients with sleep disorder:a rs-fMRI study combined with machine learning analysis1Xin-Huangpu Joint Innovation Institute of Chinese Medicine in Guangdong Province, Guangzhou, China, 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China, 3Guangzhou University of Chinese Medicine, Guangzhou, China, 4The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China, 5Philips Healthcare, Guangzhou, China
Synopsis
Keywords: fMRI (resting state), Kidney
The prevalence of SD is high in chronic kidney disorder patients who suffering maintenance hemodialysis whereas little is known about the neuropathologic mechanism. We investigated the ALFF alterations between MHDSD patients and HCs and those meaningful features were used for constructing discriminating model based the SVM algorithm to classify the MHDSD patients. We found the aberrant spontaneous activities in the DMN, AN, CEN, and VIN in MHDSD patients. Additionally, the classifier indicated the discriminative ALFF features in the above regions demonstrated good performance. This will contribute to well understanding the neuropathological mechanism and seeking biomarkers for discrimination in MHDSD patients.Introduction
As the first choice for patients with kidney failure, although maintenance hemodialysis (MHD) is lifesaving1, patients are usually accompanied by multiple complications especially maintenance hemodialysis with sleep disorder (MHDSD)2-5, which severely affects the life quality and disease prognosis3-4,6. Given the high frequency and burden to a life of the disease, a compelling need exists to understand the pathological mechanism in patients with MHDSD which is unclear at present. As known, brain function changes may occur before any neurological symptoms are observed. Therefore, it is necessary to investigate the imaging biomarkers to explore the pathogenesis for further diagnosis and treatment using resting-state functional magnetic resonance imaging (rs-fMRI).Method
A total of 30 MHDSD patients and 30 age-, gender- matched healthy controls (HC) were recruited to complete rs-fMRI scanning. All of the patients undergoing maintenance hemodialysis were diagnosed with sleep disorders based on the Fifth Edition of the American Diagnostic and Statistical Manual of Mental Disorders criteria. Functional magnetic resonance imaging data were acquired using MAGNETOM Verio 3T MR scanner (Simens Healthcare, Erlangen, Germany) with a 24-channel phased-array head coil. All subjects participated in identical MRI scanning sessions including a resting-state functional MRI scan using gradient-echo planner imaging (EPI) sequences and a T1-weighted high-resolution structural scan. The corresponding parameters are as follows:(1)EPI sequences: repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, field of view (FOV) = 224 mm × 224 mm, matrix = 64 × 64, flip angle = 90◦, slice thickness = 3.5 mm, interslice gap = 0.7 mm, 31 axial slices paralleled, and 240-time points; (2) T1-weighted sequences: TR = 1,900 ms, TE = 2.27 ms, flip angle = 9◦, FOV = 256 mm × 256 mm, matrix = 256 × 256, and slice thickness = 1.0 mm. The resting-state functional data were processed using the specialized toolbox for Data Processing and Analysis of Brain Imaging 3.0. The amplitude of low frequency fluctuation (ALFF) was used to reflect the brain function by measuring spontaneous neuronal activity, and the differences between MHDSD patients and HCs were detected using a two-sample t-test. Moreover, a partial correlation analysis was performed between the intergroup ALFF differences and clinical variables. Further, we extracted the distinct ALFF features as masks for constructing the discriminating model by a support vector machine (SVM) to classify the MHDSD patients. The performance of the classifier was assessed by accuracy, sensitivity, and specificity.Result
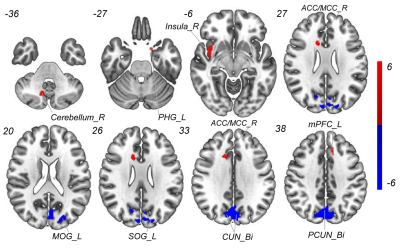
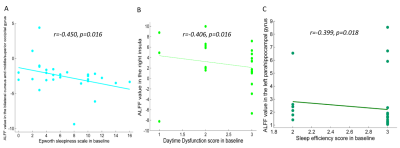
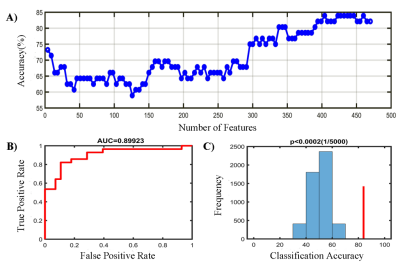
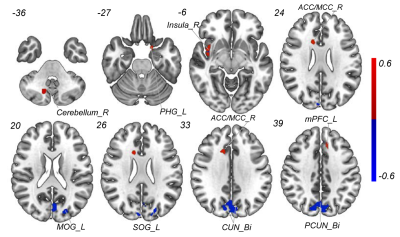
Compared with HCs, patients with MHDSD exhibited increased ALFF in the right insula, right anterior/middle cingulate cortex (ACC/MCC), left medial prefrontal cortex (mPFC), left parahippocampal gyrus(PHG), and right cerebellum, but decreased in bilateral cuneus (CUN), bilateral precuneus (PCUN), left middle occipital gyrus (MOG) and superior occipital gyrus (SOG). And the correlation results showed that the decreased ALFF value in the bilateral CUN and MOG/SOG was negatively associated with ESS scores (r=-0.450, p=0.016), the increased ALFF value in the right insula was negatively associated with daytime dysfunction score in MHDSD patients(r=-0.406, p=0.016), and the increased ALFF in left PHG was also negatively associated with sleep efficiency score (r=-0.399, p=0.018) in MHDSD patients. Furthermore, in the SVM model, the classifier indicated the discriminative ALFF features in the above brain regions demonstrated good performance with a mean accuracy of 83. 9%, sensitivity of 85.7%, and specificity of 82.1% (p<0.002).Discussion
To the best of our knowledge, this is the first study to reveal the underlying neuropathological mechanism of MHDSD and explore specific neuroimaging biomarkers to discriminate patients with MHDSD using the SVM model combined with feature selection of F-Score. In this study, compared with pairwise matched HCs, we found that MHDSD patients exhibited increased ALFF in the right insula, right ACC/MCC, right cerebellum, left mPFC, and left PHG, but decreased in bilateral CUN/PCUN and left MOG/SOG, mainly involved in the default mode network (DMN), affective network (AN), cerebellum network (CEN), and visual network (VIN) which could be possible pathologic mechanism. Additionally, the subsequent MVPA results demonstrated that the above meaningful ALFF features served as reliable biomarkers to discriminate MHDSD patients with high AUC (90.0%), sensitivity (85.7%), specificity (82.1%), and mean accuracy (83. 9%) in classifying MHDSD. Therefore, we deduced that the specific ALFF features might contribute to well understanding the pathophysiology of MHDSD and the discrimination of MHDSD anterior to clinical symptoms.Conclusion
On one hand, this study revealed the underlying pathophysiological mechanism of MHDSD by exploring imaging biomarkers which are mainly involved in the default mode network (DMN), affective network (AN), cerebellum network (CEN), and visual network (VIN). On the other hand, the machine learning analysis preliminarily demonstrated ALFF values at baseline in the above networks are the potential targets with good performance for discriminating MHDSD patients. This will contribute to well understanding the neuropathological mechanism and seeking specific imaging biomarkers for discrimination in patients with MHDSD.Acknowledgements
We would like to acknowledge the generous support and contribution of all our trial participants.References
1.Cukor D, Unruh M, McCurry SM, et al. The challenge of insomnia for patients on haemodialysis. Nat Rev Nephrol. 2021;17(3):147-148.
2.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057-1064.
3.Flythe JE, Dorough A, Narendra JH, et al. Perspectives on symptom experiences and symptom reporting among individuals on hemodialysis. Nephrol Dial Transplant. 2018;33(10):1842-1852.
4.Anand S, Johansen KL, Grimes B, et al. Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: the comprehensive dialysis study. Hemodial Int. 2013;17(1):50-58.
5.Roumelioti, M. E. Argyropoulos, C. P. & Unruh, M. L. in Psychosocial Aspects of Chronic Kidney Disease (eds Cukor, D., Cohen, S. & Kimmel, P.) 183–212 (Academic Press, 2020).
6.Cox KJ, Parshall MB, Hernandez SHA, et al. Symptoms among patients receiving in-center hemodialysis: A qualitative study. Hemodial Int. 2017;21(4):524-533.
Figures