2707
A universal B1 shim for the human cerebellum1Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 2Computational Cognitive Neuroscience and Neuroimaging, Netherlands Institute for Neuroscience, Amsterdam, Netherlands
Synopsis
Keywords: Data Acquisition, Shims, B1 shim
The human cerebellum is underexplored in-vivo due to a lack of acquisition methods that effectively portray it. Its dense architecture necessitates high resolution, which benefits from 7T imaging. Unfortunately, at higher field strengths, images suffer from severely destructive B1-interference with typical coil geometries. Subject-optimized parallel-transmit approaches can mitigate B1 artifacts but increase scan-time. We designed a universal B1-shim specific to the cerebellum and immediately applicable across individuals for high-resolution 7T fMRI. We demonstrate improvements in tSNR and functional responses in cerebellar ROIs during a hand-motor task compared to a quadrature mode shim setting.
Introduction
The cerebellum is important across the cognitive domain3,4, particularly for sensorimotor functions1,2. Despite its importance, the human cerebellum is underexplored in-vivo due to insufficient acquisition methods: Its dense architecture necessitates high resolution which benefits from ultra-high-field(7T) imaging. However, the higher 7T-RF-wavelength and the cerebellum’s lower-brain location result in destructive B1-interference with typical coil geometries, greatly reducing cerebellar image quality, especially in the posterior lobe, where function is particularly underexplored2,5. Dedicated cerebellar coil hardware can improve sensitivity in the cerebellum, but is impractical for wholebrain applications6. Alternatively, parallel transmit systems(pTx) allow for participant-specific tuning of each transmit channel, greatly improving the B1-field homogeneity in specific target ROIs7. However, person-specific B1-optimization requires B1-map acquisition and ROI-selection, thus increasing scan-time. Here, we examined if a universal B1-shim can be designed for the human cerebellum at 7T and assess its performance for cerebellar (functional) imaging.Methods
16 healthy volunteers (ages:21-42) were scanned using a 7T-Achieva with an 8Tx/32Rx (Nova Medical,USA) whole-head coil. For all participants, individual-channel B1+ fields were calculated, from the acquisition of a quadrature-mode DREAM B1+ map (FOV=192x60x192mm3, voxel-size=3.5 mm isotropic, TR/TE=6/3ms, flip-angle=7°) and a spoiled-gradient-echo acquired for each transmit-channel separately (FOV=192x60x192mm3,voxel-size=3.5mm, TR/TE=8ms/1.97 ms, flip-angle=1.5°). Optimal phase settings were calculated to minimise the cost function sd(B1+cerebellum)/mean(B1+cerebellum)2 in the cerebellum using the MRCodeTool (Tesla Dynamic Coils, Netherlands). The median phase settings of the first 12 participants for each transmit-channel were calculated (Fig-1D). For the remaining 4 participants, a whole-head MP2RAGE (voxel-size=1mm isotropic, TR/TE/TRvolume=6.2/2.3/5500ms,TI1/TI2=800ms/2700ms,α=7°/5°, FOV=352x352x109) was acquired. A 3D-EPI covering the cerebellum (Fig-1B, 1mm-isotropic, TR/TE=3288ms/21ms, SENSEAP/RL =2.6/3.27, FOV=192x60x192mm, flip-angle=20°) was recorded while a motor task was performed (right-hand flex:10s-ON 10s-OFF, Fig-1C). For one participant, 50 additional volumes were acquired during fixation for temporal-SNR calculations. All acquisitions were repeated with three different B1-shimming modes: quadrature, the personalised B1-shim and the universal B1-shim calculated from the median of the 12 participants. The acquisition order was pseudo-randomised across participants to account for task habituation. FMRI data were motion/distortion-corrected and a GLM (flexing>rest, z>3.1 p<0.05, 2mm-smoothing) was fitted for each run(FSL8). Cerebellar grey and white-matter segmentation was performed using SPM and SUIT9 . White-matter segmentations were used to assess image intensity in the anatomical images. Anatomical images were warped into MNI-space and the King cerebellar atlas for right-hand motor function10 was brought to the functional space of each participant, creating two ROIs in the right posterior and anterior lobes. For each run, the cluster size of the largest cluster in the right-hand motor ROIs was obtained. Temporal-SNR maps were calculated for all functional acquisitions (mean/temporal standard deviation).Results
B1The phase-offsets to individually-optimize the cerebellar B1-distribution were largely consistent across individuals(MEDSD=16.2°,Fig-1D), implying that a median can represent the group and a universal-cerebellar B1-shim (stars) can be found.
TSNR
Figure-2 shows the tSNR within the posterior and anterior ROI across participants for the three shimming approaches. In both ROIs, the median tSNR from the universal shim (Anterior: Med=46.0,IQR=21.37, Posterior: Med=44.1,IQR=15.73) was similar to the personalized (Anterior: Med=44.5,IQR=19.08, Posterior: Med=44.7,IQR=14.45) and higher than the quadrature mode (Anterior: Med=43.0,IQR=19.14, Posterior: Med=34.3,IQR=12.24). Visually, the tSNR of the quadrature shim is markedly lower compared to the personalized and universal shim, specifically in the region of the motor task ROIs (Fig-3).
Anatomical
The T1-weighted images from the MP2RAGE acquisition showed a more homogeneous signal intensity when using the personalised (MED =3342, SD =355.4) and universal (MED=3317, SD=387.9) cerebellar B1-shims compared to quadrature mode (MED=3216, SD=500.2), and a narrower signal-distribution in the cerebellar white-matter(Fig-4).
Cerebellar responses
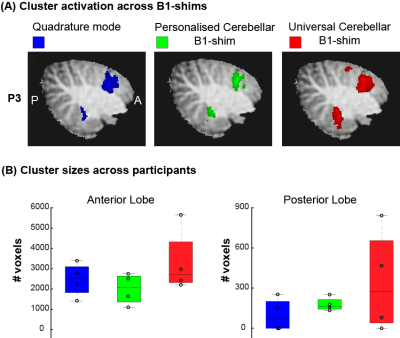
The motor task resulted in significant(Z>3.1) activation in the cerebellum in all participants for all shimming options (Fig-5A). Two participants showed no significant activation in the posterior lobe for the quadrature shim, one participant showed no significant activation in the posterior lobe for the universal shim (Fig-5B). On average, cluster sizes were larger using the personalised (Anterior:MED=2074,IQR=1275.5, Posterior:MED=164.5,IQR=71.5) and universal shim (Anterior:MED=2709,IQR=2002, Posterior:MED=273.5,IQR=614.5) than for the quadrature mode (Anterior:MED=2514,IQR=1278.5, Posterior: MED=75,IQR=201), especially in the posterior lobe.
Discussion
The universal B1-shim tSNR values were comparable to the personalized B1-shim and consistently higher compared to the quadrature mode tSNR in both functional ROIs, suggesting universal B1-shims can efficiently reduce cerebellar B1-inhomogeneities.Cerebellar functional responses were similar between universal and personalised B1-shimming approaches, showing similar cluster sizes for most participants. The posterior lobe showed on average less activation using the quadrature mode, as might also be expected from the lower tSNR. For further counterbalancing, a larger participant pool is required to increase statistical power when comparing shimming approaches. Anatomical acquisitions presented improved cerebellar signal in the universal and personalised B1-shim.
Compared to personalized B1-shim, the time-savings achieved with the universal B1-shim are significant, up to 10-minutes per participant. Other approaches like universal pulses need to be tailored to the acquisition, while our approach flexibly translates from whole-head anatomy, to slab-selective fMRI.
Conclusion
We designed a universal B1-shim approach specific to the cerebellum at 7T and demonstrate improvements in high-resolution cerebellar fMRI in both tSNR and detection of functional responses to a hand-motor task compared to quadrature mode acquisitions.Acknowledgements
No acknowledgement found.References
1. Boillat, Y., Bazin, P. L. & van der Zwaag, W. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. Neuroimage 211, (2020).
2. Van der Zwaag, W. et al. Digit somatotopy in the human cerebellum: A 7T fMRI study. Neuroimage 67, 354–362 (2013).
3. Guell, X., Schmahmann, J. D., Gabrieli, J. D. E. & Ghosh, S. S. Functional gradients of the cerebellum. Elife 7, (2018).
4. Xue, A. et al. The detailed organization of the human cerebellum estimated by intrinsic functional connectivity within the individual. J. Neurophysiol. 125, 358–384 (2021).
5. Wiestler, T., McGonigle, D. J. & Diedrichsen, J. Integration of sensory and motor representations of single fingers in the human cerebellum. J. Neurophysiol. 105, 3042–3053 (2011).
6. Priovoulos, N. et al. A local multi-transmit coil combined with a high-density receive array for cerebellar fMRI at 7 T. NMR Biomed. 34, (2021).
7. Berrington, A. et al. Calibration-free regional RF shims for MR spectroscopy. doi:10.1101/2020.07.24.20161141.
8. Jenkinson, M., Beckmann, CF., Behrens, EJT., Woolrich, M., & Smith, SM., FSL. Neuroimage 62, 782–790 (2012).
9. Diedrichsen, J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138 (2006).
10. King, M., Hernandez-Castillo, C. R., Poldrack, R. A., Ivry, R. B. & Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 22, (2019).
Figures

Figure1: Methods: acquisition set up and task design (A) B1 inhomogeneity in the cerebellum in quadrature mode highlighted with white box. (B) Sagittal and coronal view of the MP2RAGE anatomical acquisition, overlayed with the EPI FOV presented by the black box. (C) Timeline of the flexing task. ON blocks represent flexing of the right hand. (D) Phase offsets of the personalised cerebellar B1-shim of 12 participants for each transmit channel (dots) and the median phases across group (stars). (Median standard deviation across transmit channels=16,2°)
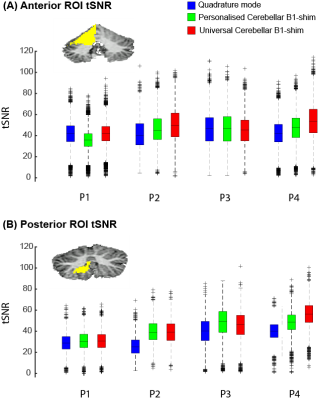
Figure 2: TSNR for all participants in two ROIs: King atlas right hand motor response in the anterior lobe and the posterior lobe. Note that in both ROIs, quadrature mode median tSNR is lower (Ant: Med=43.0 IQR=19.14, Post: Med=34.3 IQR=12.24) compared to the personalised (Ant: Med=44.5 IQR=19.08, Post: Med=44.7 IQR=14.45) and universal shim (Ant: Med=46.0 IQR=21.37, Post: Med=44.1 IQR=15.73).
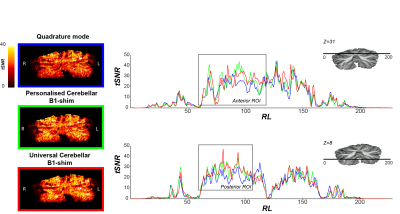
Figure 3: TSNR maps (left) and profiles (right) for participant 4 for each functional run with different B1-shim, Z=31: Anterior lobe and Z=8: Posterior lobe. Note that tSNR values are consistently lower for the quadrature mode compared to the universal cerebellar B1-shim and the personalised cerebellar B1-shim in the functional ROIs, here indicated by a grey box.
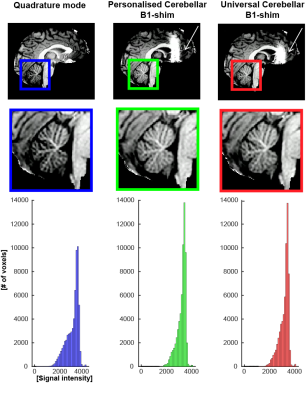
Figure 4: Top: T1w images from the MP2RAGE acquisition for each B1-shim. Note the enhanced signal in the cerebellar area in the personalised and universal cerebellar B1-shims. The area of B1-cancellation in the frontal lobe is also clearly visible in this slice (white arrows). Bottom: the histogram presents signal distribution in the cerebellar white matter, Note the narrower signal intensities distribution for both cerebellar shims.