2693
Patient-specific Local SAR estimation by combined field mapping and deep-learning method1Lauterbur Research Center for Biomedical Imaging, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province, Shenzhen, China, 3United Imaging Research Institute of Innovative Medical Equipment, Shenzhen, China, 4The University of Hong Kong, Hongkong, China, 5Biomedical Engineering, State University of New York at Buffalo, Buffalo, NY, United States
Synopsis
Keywords: Safety, Safety
A method is proposed for real time patient-specific local SAR estimation based on B1 field mapping and machine-learning. The axial component of RF E-field is estimated by electric property tomography (EPT) method from B1 field, and the transversal component of RF E-field is predicted by a cycleGAN model trained with EM simulation input data. The safety factor of peak local SAR estimation is analyzed for a large set of random transmit weighting factors and the feasibility of the method is discussed.Introduction
Despite its attraction in SNR, resolution, imaging contrast, and parallel imaging performance, ultra-high field (UHF) magnetic resonance imaging suffers from major challenges in RF safety assurance1. High RF frequency leads to increased whole-body SAR levels and uncertainty in the position and value of peak local SAR. And the use of local parallel transmit array hardware also adds to the complexity of local SAR estimation.Calculation of local SAR requires information on the E field distribution, tissue conductivity, and density, none of which are directly measurable for each specific patient in the MRI scan scenario. Early attempts in estimating local SAR used a series of pre-defined patient models to obtain field results through EM simulation2. However, the actual patient under scan may differ in body size and position, which leads to estimation errors that must be covered by an additional safety factor. Patient-specific modeling is proposed to obtain realistic patient models3. But EM simulation is time-consuming and not suitable for a real-time patient pre-scan procedure.
Previous methods used electric-property tomography (EPT) methods4 and machine-learning methods5 to achieve real-time local SAR estimation. However, both methods are based on the assumption that the z-component of the E field is dominant in the region of interest.
Methods
This work is based on an 8-channel volumetric transmit coil6 installed on the 5.0T MR scanner developed by United Imaging Healthcare. The transmit coil is modeled in Sim4Life (ZMT Zurich MedTech AG) with the 15 human models of Virtual Family and positioned in 10 landmarks from head to ankle.The z-component of the E field is estimated by the EPT method as shown below 4:
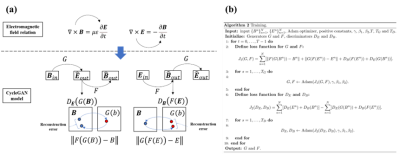
$$E_z=-(\frac{\partial B_1^+}{\partial x}-j\frac{\partial B_1^+}{\partial x}-\frac{\partial B_1^-*}{\partial x}-j\frac{\partial B_1^-*}{\partial y})/\omega \mu _0 \varepsilon_0$$
The x and y components of the E field are estimated with a deep-learning method. A cycleGAN model is constructed to map the Bx and By components to Ex and Ey components. The training set comprises E and B field data of the 8 transmit channels in the first 7 models positioned at 10 landmarks. The typical volumetric data size of one transmit channel covers 120*75*200 voxels with a uniform cubic size of 5mm. The total training set has over 100’000 effective transversal frames. The remaining 8 models are used as the test data sets.
The safety factor of the peak local SAR estimation method is evaluated by statistical analysis of 100 sets of randomly generated transmit weighting factors. In each transmit mode, the E field is estimated by different combinations of E vector estimation, and the final 10g SAR is calculated. The peak 10g SAR is found in both estimated E dataset and the ground-truth dataset. The safety factor is defined as the maximum ratio of true and estimated peak SAR in all transmit modes.
$$SF = \max\left( \frac{\text{pSA}R_{10g}^{\text{true}}}{\text{pSA}R_{10g}^{\text{estimate}}} \right)$$
Results
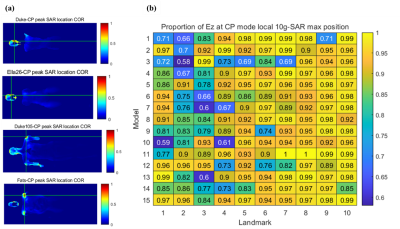
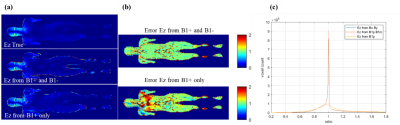
The E field vector components in 3 dimensions are analyzed to evaluate the peak local SAR estimation error with the assumption of E field z-component dominance. In Figure 2 (a), the peak SAR location is found with the head region for model Duke and Ella26, and near the shoulder for model Duke105 and Fats with larger body sizes. In Figure 2 (b), the proportion of the z-component of the E field in the peak SAR location is shown for all models and landmarks. It is shown that various human models and landmarks have a peak local SAR location with substantial transversal E field components.The result of the EPT method is shown in Figure 3. Estimation of Ez component by full information of both B1+ and B1- yield highly accurate results compared with ground-truth data. An error standard deviation of less than 5% is achieved. The estimation of Ez by B1+ only shows a higher spread error.
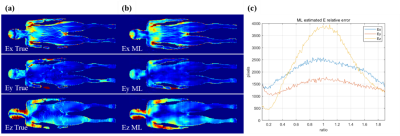
The E field output in all 3 dimensions by the trained cycleGAN model has a similar pattern compared with the ground-truth data. The distribution of the ratio is centered near 1. The Ez component has a narrower spread than both Ex and Ey components, showing smaller estimation errors.
The safety factor with different combinations of E vector estimation is shown in Figure 5. In Figure 5(a), the omission of the Ex and Ey component lead to large estimation errors of peak local SAR and impractical safety factor. The deep-learning based Ex and Ey component reduced the safety factor to a reasonable value as shown in Figure 5(b). The EPT-based Ez component with ground-truth Ex and Ey components yields the most accurate peak local SAR estimation.
Discussions and Conclusions
It is shown that the EPT approximation of the Ez component dominance may lead to estimation error for various human models and transmit modes. In the case of patient proximity to the transmit coil array, or in body parts with RF current return paths, the transversal component of the E field starts to play a part in average SAR and leads to large estimation errors. Estimation of transversal E field components by deep-learning model can substantially improve the estimation accuracy. However, the deep-learning model is based on the specific transmit coil array design of the 5.0T system, and other configuration of transmit coils needs further investigation.Acknowledgements
This work is supported by National Key Research and Development Program of China, 2021YFE0204400; the Strategic Priority Research Program of Chinese Academy of Sciences, XDB25000000; National Natural Science Foundation of China, U22A20344; Youth Innovation Promotion Association of CAS No. Y2021098; Key Laboratory Project of Guangdong Province, 2020B1212060051; Shenzhen city grant, RCYX20200714114735123.References
1. Fiedler TM, Ladd ME, Bitz AK. SAR Simulations & Safety. Neuroimage. 2018;168:33-58. doi:10.1016/j.neuroimage.2017.03.035
2. Murbach M, Neufeld E, Cabot E, et al. Virtual Population-Based Assessment of the Impact of 3 Tesla Radiofrequency Shimming and Thermoregulation on Safety and B 1 1 Uniformity. 2015;00:1-12. doi:10.1002/mrm.25986
3. Homann H, Börnert P, Eggers H, Nehrke K, Dössel O, Graesslin I. Toward individualized SAR models and in vivo validation. Magn Reson Med. 2011;66(6):1767-1776. doi:10.1002/mrm.22948
4. Zhang X, Schmitter S, Moortele P françois Van De, et al. From Complex B1 Mapping to Local SAR Estimation for Human Brain MR Imaging Using Multi-Channel Transceiver Coil at 7T. IEEE Trans Med Imaging. 2013;32(6):1058-1067. doi:10.1109/TMI.2013.2251653
5. Meliadò EF, Raaijmakers AJE, Sbrizzi A, et al. A deep learning method for image-based subject-specific local SAR assessment. Magn Reson Med. 2020;83(2):695-711. doi:10.1002/mrm.27948
6. Fang F, Luo W, Gong J, Zhang R, Wei Z, Li Y. An 8-channel transmit loop array for body imaging at 5T. 2020;46(1):24-26.
Figures