2684
Identify the paraventricular thalamic nucleus in humans using a structural connectivity approach1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Faculty of Health Science, Juntendo University, Chiba, Japan, 3Department of Psychiatry, Juntendo University, Tokyo, Japan, 4Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan
Synopsis
Keywords: Brain Connectivity, Brain Connectivity
We attempted to identify the paraventricular thalamic nucleus (PVT), which is known to regulate emotion, motivation, stress, and drug- and alcohol-related behaviors in humans. We used data-driven connectivity profiles obtained using probabilistic tractography and a k-means clustering method with diffusion-weighted imaging data. We consistently identified an anatomical connectivity-based parcellation of the PVT in two independent cohorts that included 601 healthy subjects. Furthermore, we discerned the specific structural pattern of the PVT, which agreed with findings from animal studies. Finally, we noted significant correlations between PVT structural and functional connectivity with the limbic structures and drug-, nicotine-, or alcohol-related scores.
INTRODUCTION
The paraventricular nucleus of the thalamus (PVT) is a small structure located medial to the magnocellular subdivision of the mediodorsal thalamus (MDmc). The PVT is an important hub of the limbic network and is the only thalamic nucleus connected to the group of limbic structures comprising the amygdala, bed nucleus of the stria terminalis (BNST), and nucleus accumbens (NAcc)1. In animals, the PVT has been shown to be closely related to emotional and motivational processes, stress regulation, and drug- and alcohol-related behaviors2. However, identifying the PVT in humans is challenging.We aimed to identify the PVT based on data-driven connectivity profiles obtained using probabilistic tractography and a k-means clustering method3 with diffusion-weighted imaging (DWI) data. We further investigated the associations between PVT structural and functional connectivity with limbic structures and drug-, nicotine-, and alcohol-related scores.
METHODS
Study participants and MRI data acquisitionMRI data of 502 healthy individuals from the Human Connectome Project4 and 99 healthy individuals from the UCLA Consortium for Neuropsychiatric Phenomics LA5c Study5 were assessed as the first and test cohorts, respectively (Figure 1). DWI, T1‑weighted imaging, and resting state functional MRI (rs-fMRI) data were obtained using 3T-MRI scanners with acquisition parameters (Figure 2). Alcohol, tobacco, and drug-related scores were collected from the first cohort.
Structural connectivity-based segmentation of the PVT
Probabilistic tractography was performed using probabilistic tracking with crossing fibers (PROBTRACKX) based on the Bayesian Estimation of Diffusion Parameters (BEDPOSTX)6. The PVT has not yet been defined in any existing MRI atlases. However, by incorporating the recently proposed FreeSurfer-based thalamic nuclei parcellation7 and a previous autopsy brain study8, it was suggested that the PVT is part of the MDmc9. Therefore, probabilistic fiber tracking was performed in each subject using FreeSurfer’s MDmc as the seed region and the cortical and subcortical areas (except the thalamus and BNST)10 as the target regions. Subsequently, the MDmc was segmented into two regions using a k‑means clustering algorithm3 based on the frequency matrix of fibers originating from each seed voxel in the MDmc and reaching the specific ipsilateral target voxels.
Structural connectivity patterns through the PVT
Based on the results of k-means clustering, the MDmc was segmented into dorsomedial and ventrolateral regions, and the former was defined as the PVT considering the known anatomical position8. The structural connectivity patterns of each region were calculated as the ratio of the number of streamlines from each cluster to the target regions and the number of projections from the entire MDmc to the target regions. Furthermore, the number of streamlines of PVT-NAcc, PVT-BNST, and PVT-amygdala were calculated and correlated with alcohol, nicotine, and drug-related scores. The number of streamlines was divided by PVT volume to eliminate the effect of atrophic changes in the thalamus.
fMRI processing
The rs-fMRI data of the part of first cohort (randomly chosen 122 subject) were evaluated using the CONN toolbox11 to assess the functional association between the PVT and the NAcc, BNST, or amygdala. The Fisher-transformed bivariate correlation coefficient was applied as ROI-to-ROI functional connectivity.
Statistical analysis
Correlations between PVT structural and functional connectivity and alcohol, tobacco, and drug-related scores were calculated using partial correlation tests after adjusting for age, sex, and intracranial volume (a: P < 0.05).
RESULTS
We were able to segment the MDmc into dorsomedial and ventrolateral regions consistently in both cohorts (Figure 3). The tracts originating from the dorsomedial part of the MDmc predominantly projected into the limbic areas, including the amygdala, NAcc, BNST, and anterior cingulate cortex. Thus, this area most likely represents the PVT (Figure 4). The ventrolateral regions of the MDmc corresponded to the anatomical part of the MDmc.We observed significant associations between: (1) PVT-BNST structural connectivity and DSM-IV criteria for alcohol dependence/frequency of any alcohol use in the past 12 months/times of usage of any illicit drugs, except marijuana/times of usage of marijuana; (2) PVT-NAcc and frequency of any alcohol use in the past 12 months/times of usage of marijuana (Figure 5A). Significant associations were also demonstrated between: (1) PVT-BNST functional connectivity and DSM-IV criteria alcohol dependence or Fagerstrom Heavy Smoking Index; (2) PVT-NAcc functional connectivity and Fagerstrom Test for Nicotine Dependence score (Figure 5B).
DISCUSSION
We successfully identified a structure likely to be the PVT using a data-driven connectivity-based brain parcellation procedure based on anatomical connectivity patterns obtained from DWI and probabilistic tractography. The structural pattern of the identified PVT was consistent with the findings from animal studies1,2. The PVT showed strong connections with the limbic structures, including the amygdala, NAcc, BNST, and anterior cingulate cortex. Indeed, the PVT is known to be the only thalamic nucleus with connections to the limbic system1. Furthermore, we observed significant correlations between PVT structural or functional connectivity with the limbic structures and drug, nicotine, or alcohol-related scores. Consistent with our findings, recent animal studies1,2 have suggested the essential roles of PVT-BNST and PVT-NAcc in drug, nicotine, and alcohol-related behaviors. In conclusion, structural connectivity features obtained from probabilistic diffusion tractography enable the segmentation of the PVT. Furthermore, assessment of connectivity between the PVT and limbic structures may aid in evaluating the neural basis of alcohol, nicotine, and drug addiction.Acknowledgements
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This research was supported by Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (JSPS KAKENHI; Grant Numbers: 21K15851, 19K17244, and 18H02772) , Brain/MINDS Beyond program (grant no. JP19dm0307101) of the Japan Agency for Medical Research and Development (AMED), AMED under grant number JP21wm0425006, a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan, and the Juntendo Research Branding Project.
References
1. Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci 2014; 8: 73.
2. Hartmann MC, Pleil KE. Circuit and neuropeptide mechanisms of the paraventricular thalamus across stages of alcohol and drug use. Neuropharmacology 2021; 198: 108748.
3. Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cerebral cortex 2016; 26(8): 3508-26.
4. Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013; 80: 105-24.
5. Gorgolewski KJ, Durnez J, Poldrack RA. Preprocessed Consortium for Neuropsychiatric Phenomics dataset. F1000Res 2017; 6: 1262.
6. Jbabdi S, Sotiropoulos SN, Savio AM, Graña M, Behrens TE. Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magn Reson Med 2012; 68(6): 1846-55.
7. Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 2018; 183: 314-26.
8. Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 1989; 14(1): 1-34.
9. Shin KJ, Lee HJ, Park KM. Alterations of individual thalamic nuclei volumes in patients with migraine. J Headache Pain 2019; 20(1): 112.
10. Neudorfer C, Germann J, Elias GJB, Gramer R, Boutet A, Lozano AM. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci Data 2020; 7(1): 305.
11. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2(3): 125-41.
Figures
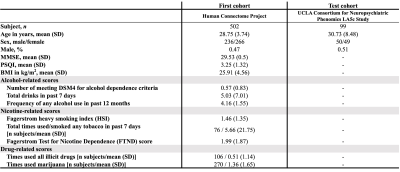
Figure 1. Demographic characteristics of study participants
Abbreviations: SD, standard deviation; MMSE, Mini-Mental State Examination; PSQI, Pittsburgh Sleep Quality Index; BMI, body mass index
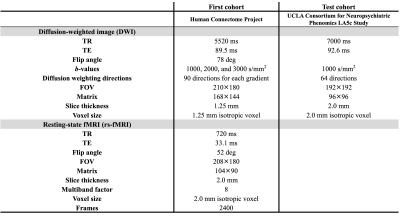
Figure 2. MRI acquisition parameters
Diffusion-weighted images (DWI) were obtained from both cohorts; however, resting-state functional MRI (rs-fMRI) data were only available for cohort 1. Note: All DWI data were corrected for susceptibility, eddy current-induced geometric distortions, and intervolume subject motion with the topup and eddy tools implemented in FSL. All rs-fMRI data were corrected for motion and linear and quadratic trends using the CONN toolbox.
Abbreviations: TR, repetition time; TE, echo time, FOV, field of view
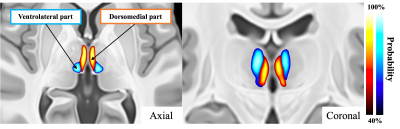
Figure 3. Segmentation of the magnocellular subdivision of the mediodorsal thalamus (MDmc)
The MDmc was consistently segmented into dorsomedial (orange) and ventrolateral (blue) parts in both cohorts. The figures show the segmented MDmc probability map generated from the first cohort in the axial and coronal planes (n = 502).
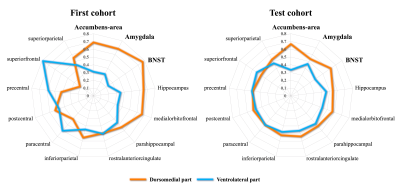
Figure 4. Structural connectivity patterns of tracts originating from dorsomedial and ventrolateral parts of the MDmc
The tracts originating from the dorsomedial part of the MDmc predominantly projected into the limbic areas (orange line) in the first cohort. A similar trend of projection pattern from the dorsomedial part of the MDmc was observed in the test cohort. Referring to previous autopsy brain studies, the dorsomedial part of the MDmc most likely represents the PVT.
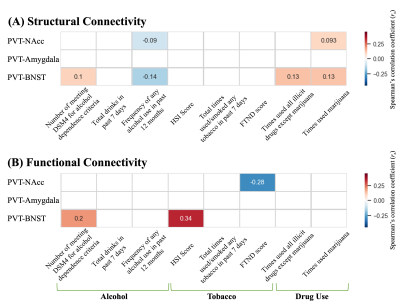
Figure 5. The associations between PVT structural (A) and functional (B) connectivity with the limbic structures and drug-, nicotine-, and alcohol-related scores
Partial Spearman’s correlation coefficient (rs) is depicted using color magnitude (red, positive; blue, negative) and was adjusted for age, sex, and intracranial volume.
Abbreviations: FTND, Fagerstrom Test for Nicotine Dependence; HIS, Fagerstrom Heavy Smoking Index