2681
Brain connectome-based imaging markers for identifiable signature of migraine with and without aura1Department of Radiology, Nanjing first hospital,Nanjing Medical University, Nanjing, China, 2Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Brain Connectivity, Machine Learning/Artificial Intelligence
In comparison with migraine without aura (MwoA), migraine with aura (MwA) has its own characteristics in symptom, pathological mechanism, treatment and prognosis. In this study, we conducted connectome-based analysis to capture brain connectivity markers that would show identifiable signature of MwA and MwoA, using diffusion tensor imaging and resting-state functional MRI. We found that the alterations of structural and functional connectivity strength contributed to migraine patient subtyping. The whole brain connectome-based imaging markers might provide possible evidence in understanding the heterogeneity of migraine with aura and help for patient-specific decision.Migraine headache is a neurological disorder that directly affects over one billion people in the world [1]. Around 30% migraineurs experience wide spectrum of aura, including visual, sensory, speech and/or language, motor, brainstem and retinal symptoms that precede the headache phase [2]. Migraine patients with aura (MwA) have higher ischemic stroke and cardiovascular disease risk than migraine patients without aura (MwoA) [3, 4]. Thus, aura specific therapy may help in reducing risk of aura related vascular event. Either to terminate headache attack or to prevent the next attack from happening, aura migraine subtyping based on understanding of the mechanism of the disease is preferred [5]. However, the subtyping of migraine patients into MwA and MwoA based on aura symptom may be questionable. Solid evidence other than symptom manifestation for migraine subtyping, as well as for further understanding the heterogeneity of migraine aura and its underlying mechanism is still needed. This study aimed to capture brain connectome-based imaging markers that would show identifiable signature of MwA and MwoA.
Methods
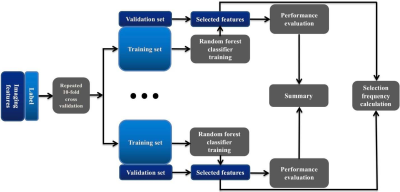
Eighty-eight migraine patients (32 MwA) and 49 healthy controls (HC) underwent a diffusion tensor imaging (echo-planar imaging, 64 weighted directions and 2 b0 images, b = 1000 s/mm2, resolution 2 × 2 × 2 mm3, TE/TR = 80 ms/8300 ms) and resting-state functional MRI (echo-planar imaging, resolution 2.75 × 2.75 × 4 mm3, TR/TE = 2000 ms/30 ms, 230 volumes in 8 min and 8 seconds, eyes closed) scanning in a 3T MRI system (uMR 780, United Imaging Healthcare, Shanghai, China). According to the Brainnetome atlas segmented the brain into 210 cortical and 36 sub-cortical subregions, ROI-based structural and functional connectivity analysis was employed to extract 60270 connectome-based imaging features. The extracted features were put into an all-relevant feature selection procedure within cross-validation loops to identify features with significant power for patient classification (Figure 1). Permutation test (permuted 1000 times) was applied to identify the features with significantly higher selection frequency than random values as MwA-related selections. And the statistical result was corrected for multiple comparisons using the false discovery rate (FDR) method for the corresponding p value. Based on selected features, the predictive ability of the random forest model constructed with previous 88 migraine patients’ sample was tested in an external sample of 32 patients (8 MwA).
Results
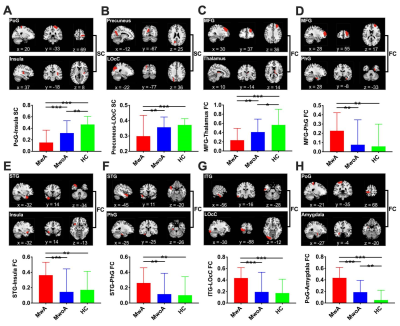
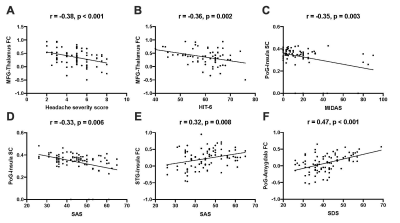
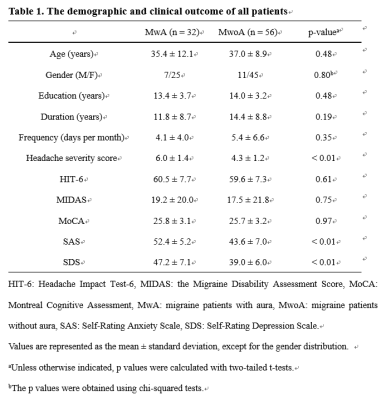
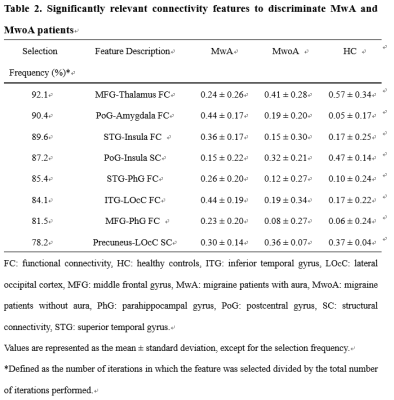
The demographic characteristics and clinical assessment of all patients were summarized in Table 1. There were no significant differences in age, gender and education between migraine patients and HCs, and also between patients with (MwA) and without aura (MwoA), using a chi-squared test for gender and two-tailed t-tests for continuous variables. The MwA group showed higher headache severity score, SAS and SDS scores compared to the MwoA group (all p values < 0.01). Eight connectivity features were identified to be significantly relevant to patient classification by permutation test (all p values < 0.001, Table 2). They all showed significant differences between MwA and MwoA, also between MwA and HC (all p values < 0.01, Figure 2). Some of these relevant features were significantly correlated with clinical rating scales in all patients (all p values < 0.01, after FDR correction; Figure 3). On basis of these features, the random forest model constructed from the training sample of 88 patients achieved an accuracy of 78.1% in the testing sample of 32 patients to identify MwA.
Discussion & Conclusion
This study applied an all-relevant feature selection algorithm to identify brain connectome-based imaging features that contribute to classify migraine into MwA and MwoA in a data-driven manner. The results indicated that eight brain connectome-based features have the identifiable power to recognize MwA. Based on these connectivity imaging features, both the accuracy and consistency of the random forest model were nearly to 80%. The reliability of this prediction model was validated by an external testing group. Comparing to nested-leave-one-out cross validation and convolutional neural network applied in single functional or structural connectivity feature studies of migraine, the all-relevant feature selection algorithm had the advantage in multiple features extraction, time saving, and in avoidance of reducing the dimensionality of feature space. The differences in functional and structural connectivity between MwA and HC, as well as between MwA and MwoA could serve as connectome-based imaging markers to distinguish MwA with promising moderate accuracy of 82.6%. We validated the 8 connectome-based image markers derived from the 88 training sample with an independent sample of migraines scanned in another MRI system, with the MwA predictive accuracy of 78.1%. Together, these imaging markers captured via forest random classifiers are reliable in identifying MwA, and this finding is replicable across an independent sample cohort and another scanner system. The model we established to identify the imaging markers of migraine with aura is robust. In summary, our present work revealed that both the higher and lower functional connectivity existed in MwA and MwoA comparing to HC. The weakened structural connectivity of MwA and MwoA was validated in our results. The identified connectome-based markers could serve as migraine subtype classifiers that contribute to understand potential mechanisms of MwA and MwoA.
Acknowledgements
This research was supported by Nanjing Science and Technology Planning Project (No. 202002056).References
1. Ashina M, Katsarava Z, Do TP, et al (2021) Migraine: epidemiology and systems of care. Lancet 397:1485–1495. https://doi.org/10.1016/S0140-6736(20)32160-7
2. Ashina M (2020) Migraine. N Engl J Med 383:1866–1876. https://doi.org/10.1056/NEJMra1915327
3. Chen D, Willis-Parker M, Lundberg GP (2020) Migraine headache: Is it only a neurological disorder? Links between migraine and cardiovascular disorders. Trends Cardiovasc Med 30:424–430. https://doi.org/10.1016/j.tcm.2019.10.005
4. Yemisci M, Eikermann-Haerter K (2019) Aura and Stroke: Relationship and what we have learnt from preclinical models. J Headache Pain 20:. https://doi.org/10.1186/s10194-019-1016-x
5. Burstein R, Noseda R, Borsook D (2015) Migraine: Multiple Processes, Complex Pathophysiology. J Neurosci 35:6619–6629. https://doi.org/10.1523/JNEUROSCI.0373-15.2015
Figures