2666
TR(acking) individuals down: Exploring the effect of temporal resolution in resting-state fingerprinting1CIMeC, University of Trento, Trento, Italy, 2Institute of Bioengineering, Center for Neuroprosthetics, EPFL, Geneva, Switzerland, 3Department of Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland, 4Institute of Bioengineering, EPFL, Lausanne, Switzerland
Synopsis
Keywords: Brain Connectivity, fMRI (resting state)
Brain fingerprints reside in timescale-specific functional connectivity of specific regions. However, the effect of acquisition’s temporal resolution on fingerprinting is unknown. Here, we manipulated repetition time (TR) in resting-state fMRI acquisitions, and observed that subject identifiability was maximized when using fast (TR = 0.5 s) or slow (TR = 3 s) protocols, and decreased with TR = 0.7, 1 or 2 s. Moreover, while high-level association areas gave the highest contribution to individual fingerprinting regardless of TR, low-level sensorimotor areas were mostly involved in discriminating between acquisitions with different TRs.Introduction
In the last few years, brain fingerprinting has emerged as an influential tool to quantify test-retest reliability in neuroimaging studies1,2,3 and to identify cognitive biomarkers in both healthy and clinical populations1,4,5,6. The idea behind is that an individual’s functional connectivity (FC) profile is sufficiently unique to be discriminated among a large group of subjects with high accuracy, even across different tasks1 and MR scanners2. Interestingly, each brain region gives a different contribution to fingerprinting1, which is maximized at specific timescales7, consistently with underlying mental processes7. This raises the issue of how acquisition timescales may affect fingerprinting. In this study, we assess the temporal features of brain fingerprints by exploring whether subject identifiability is affected by the temporal resolution at which full brain data are acquired. In particular, the contribution of whole-brain and network-specific FC to fingerprinting is investigated when resting-state fMRI (rs-fMRI) data are acquired with repetition time (TR) ranging from 0.5 to 3 seconds.Methods
Twenty healthy volunteers (10 females, age: 24±3 years) underwent five sessions of rs-fMRI in a 3T Siemens Magnetom Prisma at CIMeC (University of Trento). Acquisitions parameters for each run were identical (TE=28ms, 3mm isotropic voxels, FA=59 degree, simultaneous multislice acceleration factor=6, 56 slices), except for TR, which was manipulated such that the total scanning time was kept constant at 7.40 minutes, subsequently affecting the number of volumes (NoV): TR/NoV=0.5s/905; TR/NoV=0.7s/646; TR/NoV=1s/452; TR/NoV=2s/226; TR/NoV=3s/150.After preprocessing (comprising slice timing and head motion correction, coregistration to anatomy, band-pass filtering [0.01-0.3 Hz], nuisance regression, normalization to MNI space, spatial smoothing), data were parcellated using Glasser multimodal atlas8 and a subcortical atlas provided by the Human Connectome Project9. Pearson’s correlation between all parcels’ time courses was then calculated to create two FC matrices for each subject and each TR session: one matrix obtained from the first half of the scanning time course (test), and one matrix from the second half (retest). A Principal Component Analysis (PCA)-based reconstruction of each FC matrix for maximizing fingerprints in human connectomes1 was performed.
Pearson’s correlation between test and retest FCs resulted in an identifiability matrix, on which differential identifiability (Idiff) was computed as the difference between mean diagonal and mean off-diagonal values multiplied for 100. Idiff quantifies to what extent an individual FC profile is similar to itself between visits and, at the same time, dissimilar to other individuals FC1. Intraclass Correlation Coefficient (ICC)10 was used to assess the contribution of each connectome edge to the identifiability of subjects1 regardless of TR (subject ICC), and to the identifiability of TRs regardless of subjects (TR ICC).
Results
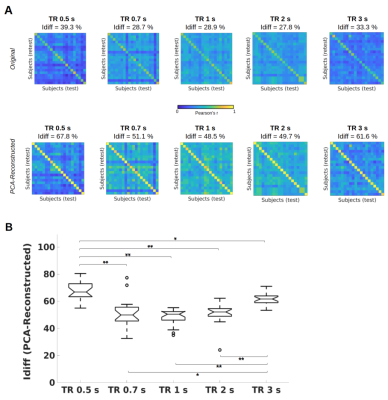
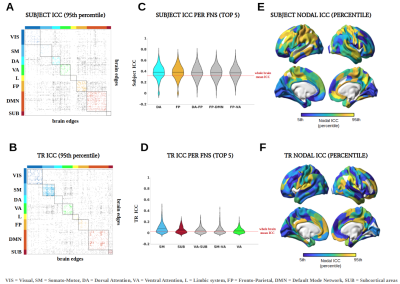
Figure 1A shows the identifiability matrices as function of TR, both with and without the optimal PCA reconstruction. Idiff was significantly higher at the optimal reconstruction (pFDR<0.001, Wilcoxon signed rank test) for all TRs. For each TR condition, a significant effect was found when comparing Idiff after reconstruction against a null distribution obtained by randomly shuffling test and retest FCs over 1000 iterations (p<001, permutation testing11). Pairwise Idiff comparisons between different TRs (Figure 1B) revealed that Idiff in TR 0.5s was significantly higher than Idiffs from all other TRs (pFDR<0.05, Wilcoxon test), and that TR 3s significantly outperformed TR 0.7, 1 and 2s in Idiff (pFDR<0.05, Wilcoxon test).Edgewise ICC showed that within- and between-network connections involving fronto-parietal, default mode, dorsal and ventral attention networks had a prominent role in identifying subjects regardless of TRs, while somatomotor and subcortical areas appeared among the top networks implicated in separating between TRs (Figure 3).
Discussion
Intrinsic FC fingerprints were successfully extracted in all TR conditions. However, subject identifiability was maximized at TR = 0.5s and TR = 3s. TR 3s can sample the BOLD signal at ∼0.16 Hz according to the Nyquist theorem, implicitly low-pass filtering respiratory ∼0.3Hz and cardiovascular ∼1Hz pulsations13 and thus again increasing test-retest reliability. Lower Idiff values at TRs = 0.7, 1, 2s may be due to relatively higher physiological noise aliasing at these intermediate sampling rates12, while at TR 0.5s data can be more efficiently denoised13.Consistent with previous studies1,7, high-level association areas where FC is less predictable on the basis of structural neural architecture14, were more prominently involved in fingerprinting. We extended these findings by showing that this effect was not impacted by the temporal resolution of the acquisition protocol. This is consistent with the interpretation that these association regions are characterized by slower intrinsic neural activity15 captured by both fast and slow signal sampling rates. On the contrary, low-level cognition areas have a higher role in separating between TRs, since their brief transient neural activity15 is registered by faster but not by slower TRs.
Conclusion
Temporal resolution in resting-state fMRI data acquisition affects fingerprinting, and consequently within-session reliability and generalizability of results. In particular, low-level somatomotor and subcortical regions are more sensitive to changes in TR. On the contrary, high-level association areas are predominantly responsible for subject identifiability regardless of TR.Acknowledgements
This study was supported by the Autonomous Province of Trento, Italy (Project: “NeuSurPlan and integrated approach to neurosurgery planning based on multimodal data") and the ISMRM 2021 Research Exchange Grant (Project: “Investigating in vivo human brain dynamic connectivity with fast fMRI”).
This work was supported by the project "Towards a reliable artificial intelligence to support decisions" (AI@TN: A proposal from the Trentino Research and Innovation System), financed by the Autonomous Province of Trento, Italy.
References
Amico, E., & Goñi, J. (2018). The quest for identifiability in human functional connectomes. Scientific reports, 8(1), 1-14.
Bari, S., Amico, E., Vike, N., Talavage, T. M., & Goñi, J. (2019). Uncovering multi-site identifiability based on resting-state functional connectomes. NeuroImage, 202, 115967.
Rajapandian, M., Amico, E., Abbas, K., Ventresca, M., & Goñi, J. (2020). Uncovering differential identifiability in network properties of human brain functional connectomes. Network Neuroscience, 4(3), 698-713.
Sorrentino, P., Rucco, R., Lardone, A., Liparoti, M., Lopez, E. T., Cavaliere, C., ... & Amico, E. (2021). Clinical connectome fingerprints of cognitive decline. NeuroImage, 238, 118253.
Svaldi, D. O., Goñi, J., Abbas, K., Amico, E., Clark, D. G., Muralidharan, C., ... & Apostolova, L. G. (2021). Optimizing differential identifiability improves connectome predictive modeling of cognitive deficits from functional connectivity in Alzheimer's disease. Human Brain Mapping, 42(11), 3500-3516.
Romano, A., Lopez, E. T., Liparoti, M., Polverino, A., Minino, R., Trojsi, F., ... & Sorrentino, P. (2022). The progressive loss of brain network fingerprints in Amyotrophic Lateral Sclerosis predicts clinical impairment. NeuroImage: Clinical, 35, 103095.
Van De Ville, D., Farouj, Y., Preti, M. G., Liégeois, R., & Amico, E. (2021). When makes you unique: temporality of the human brain fingerprint. Science advances, 7(42), eabj0751.
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., ... & Van Essen, D. C. (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171-178.
Van Essen, D. C., Smith, S. M., Barch, D. M., Behrens, T. E., Yacoub, E., Ugurbil, K., & Wu-Minn HCP Consortium. (2013). The WU-Minn human connectome project: an overview. Neuroimage, 80, 62-79.
Bartko, J. J. The Intraclass Correlation Coefficient as a Measure of Reliability. Psychol. Rep. 19, 3–11 (1966)
Sareen, E., Zahar, S., Van De Ville, D., Gupta, A., Griffa, A., & Amico, E. (2021). Exploring MEG brain fingerprints: evaluation, pitfalls, and interpretations. NeuroImage, 240, 118331.
Huotari, N., Raitamaa, L., Helakari, H., Kananen, J., Raatikainen, V., Rasila, A., ... & Korhonen, V. O. (2019). Sampling rate effects on resting state fMRI metrics. Frontiers in Neuroscience, 13, 279.
Jahanian, H., Holdsworth, S., Christen, T., Wu, H., Zhu, K., Kerr, A. B., ... & Zaharchuk, G. (2019). Advantages of short repetition time resting-state functional MRI enabled by simultaneous multi-slice imaging. Journal of Neuroscience Methods, 311, 122-132.
Preti, M. G., & Van De Ville, D. (2019). Decoupling of brain function from structure reveals regional behavioral specialization in humans. Nature communications, 10(1), 1-7.
R. Gao, R. L. van den Brink, T. Pfeffer, B. Voytek, Neuronal timescales are functionally dynamic and shaped by cortical microarchitecture. eLife 9, e61277 (2020).
B. T. T. Yeo, F. M. Krienen, J. Sepulcre, M. R. Sabuncu, D. Lashkari, M. Hollinshead, J. L. Roffman, J. W. Smoller, L. Zöllei, J. R. Polimeni, B. Fischl, H. Liu, R. L. Buckner, The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Figures