2664
Associations of the shape of subcortical brain structures with age-related neuropathologies in community-based older adults1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
Keywords: Alzheimer's Disease, Neurodegeneration, Aging, Atherosclerosis
Multiple age-related neuropathologies lead to atrophy of subcortical brain structures. However, definitive diagnosis of most of these pathologies is only possible at autopsy, complicating investigations into the effects of age-related neuropathologies on the shape of subcortical brain structures. The present work combined ex-vivo MRI and detailed pathologic assessment in a large number (N=842) of community-based older adults to study the relationship between age-related neuropathologies and the shape of subcortical brain structures. The resulting deformation patterns may be used as features in in-vivo classifiers of age-related neuropathologies.Introduction
Subcortical brain structures have important roles in cognitive and motor function and behavior. Age-related neuropathologies lead to substantial atrophy of subcortical brain regions. However, the effects of age-related neuropathologies on the shape of subcortical brain structures are not well understood because a) definitive diagnosis of most neuropathologies is only possible at autopsy and b) multiple neuropathologies often coexist in the brain of older adults. The purpose of this work was to integrate ex-vivo MRI and detailed neuropathologic examination in a large number of community-based older adults in order to make the most comprehensive investigation to-date on the association of the shape of subcortical brain structures with age related neuropathologies.Methods
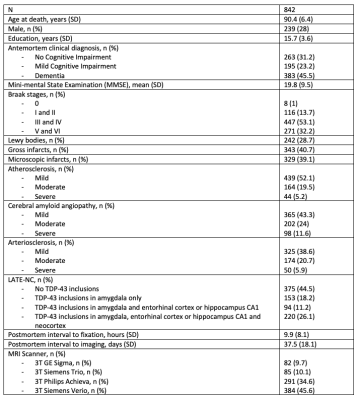
Participants and DataCerebral hemispheres from 842 older adults participating in four longitudinal, clinical-pathologic cohort studies of aging: the Rush Memory and Aging Project (MAP), the Religious Orders Study (ROS), the Minority Aging Research Study (MARS) and the Clinical Core (CC) of the Rush Alzheimer’s Disease Research Center (ADRC)1,2 (Fig.1) were included in this work. All hemispheres were imaged at room temperature while immersed in 4% formaldehyde solution using clinical 3T MRI scanners approximately 30 days postmortem. After ex-vivo MRI, each hemisphere underwent detailed neuropathologic examination by a board-certified neuropathologist (Fig.1).
Ex-vivo MR image processing
The following subcortical structures were segmented in ex-vivo T2-weighted MR images from all participants: hippocampus, amygdala, caudate, putamen, thalamus, and nucleus accumbens. Since one hemisphere was imaged for each participant, right hemispheres were mirrored to appear like left and combined with the rest of the left hemispheres in the analysis. Hemispheres were rigidly aligned and then the alignment of each subcortical structure was finetuned separately through additional rigid registration to its structure’s corresponding template.
Shape estimation
Shape analysis was performed using the spherical harmonic basis function toolbox SPHARM-PDM3. For each subcortical structure, a triangulated mesh was formed on the surface of the segmentation, and a spherical parameterization was produced by mapping the mesh to a sphere using an area-preserving and distortion-minimizing spherical mapping algorithm. The parameterization was then sampled, forming a triangulated mesh with 1002 vertices on the accumbens and amygdala surfaces, 1442 vertices on the thalamus, hippocampus and putamen surfaces, and 2252 vertices on the caudate surface. For each subcortical structure, the difference vector between a vertex on the average mesh and the corresponding vertex on a participant’s mesh was computed, for all vertices and all participants. Then, each difference vector was projected to the normal vector on the corresponding vertex of the average mesh to obtain a signed local shape difference of a participant’s subcortical structure from the average shape. The signed shape differences were used in the final statistical analysis indicating atrophy or expansion of a structure relative to the corresponding average shape.
Statistical analysis
Vertex-wise linear regression was performed for each subcortical structure, modeling the signed shape difference at each vertex (dependent variable) as a function of neuropathologies (specifically amyloid-β plaques, neurofibrillary tangles, limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC), Lewy bodies, arteriolosclerosis, atherosclerosis, gross and microscopic infarcts, and cerebral amyloid angiopathy), and controlling for age at death, sex, years of education, postmortem interval to fixation, postmortem interval to imaging, and scanner. The statistical analysis was conducted using PALM4 with more than 20 times as many permutations as the number of vertices of each structure and family-wise error rate correction. Associations were considered significant in vertices with p<0.05.
Results and Discussion
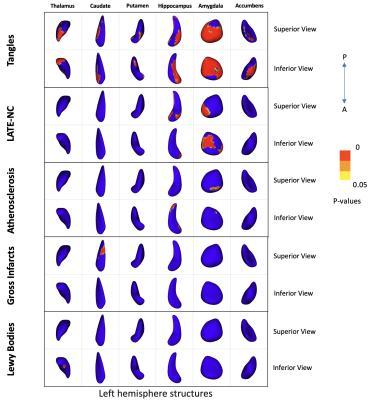
Results of shape analysis are shown in Figure 2. Tangles were associated with atrophy in all 6 subcortical structures, while LATE-NC and atherosclerosis were associated with atrophy in the hippocampus and amygdala, and gross infarcts and Lewy bodies with atrophy in the caudate and thalamus respectively. In the hippocampus, tangles were associated with inward deformation in the superior-lateral and inferior-medial aspect of the structure, LATE-NC with inward deformation in the anterior-medial and anterior-lateral aspect, and atherosclerosis in posterior-lateral and anterior-medial portion of the structure. In the amygdala, tangles were associated with inward deformation in most of the structure, LATE-NC in the superior-medial and inferior-lateral aspect of the structure, and atherosclerosis in the superior-anterior aspect. These findings for tangles and LATE-NC were in good agreement with our previous work5,6. Unique patterns of inward deformation linked to different neuropathologies were also shown in the thalamus, caudate, putamen and accumbens (Fig.2). Microscopic infarcts, arteriolosclerosis, and cerebral amyloid angiopathy did not show any significant effects on the shape of any subcortical structure. Also, no pathology was associated with outward deformation of any of the structures under study. Finally, although MRI was conducted ex-vivo, we expect the results to translate well to in-vivo, since we have previously shown that a linear relationship exists between structural brain information collected ex vivo and in vivo7.Conclusion
We present the most comprehensive MRI-pathology investigation on the association of age-related neuropathologies with the shape of subcortical brain structures in a large number of community-based older adults. Our results reveal the portions of the subcortical structures that are most vulnerable to different age-related neuropathologies. The differences in spatial patterns of the effects of various neuropathologies on the shape of subcortical brain structures may contribute towards the development of tools for in-vivo prediction of these neuropathologies.Acknowledgements
This study was supported by the following grants:
National Institute on Aging (NIA): R01AG064233, R01AG067482, R01AG017917, R01AG015819, RF1AG022018, R01AG056405, R01AG052200, P30AG010161, P30AG072975
National Institute of Neurological Disorders and Stroke (NINDS): UH2-UH3NS100599, UF1NS100599
References
1. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734-745.
2. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161-S189.
3. Styner M, Oguz I, Xu S, et al. Framework for the Statistical Shape Analysis of Brain Structures using SPHARM-PDM. Insight J. 2006;(1071):242-250.
4. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92(100):381-397.
5. Hanko V, Apple AC, Alpert KI, et al. In vivo hippocampal subfield shape related to TDP-43, amyloid beta, and tau pathologies. Neurobiol Aging. 2019;74:171-181.
6. Makkinejad N, Schneider JA, Yu J, et al. Associations of amygdala volume and shape with transactive response DNA-binding protein 43 (TDP-43) pathology in a community cohort of older adults. Neurobiol Aging. 2019;77:104-111.
7. Kotrotsou A, Bennett DA, Schneider JA, et al. Ex vivo MR volumetry of human brain hemispheres. Magn Reson Med. 2014;71(1):364-374.
Figures