2663
7T GRE Enables Detection of Subiculum Iron Deposits in AD1Stanford University, Stanford, CA, United States, 2GE Healthcare, Seattle, WA, United States, 3Weill Cornell Medical College, New York, NY, United States
Synopsis
Keywords: Alzheimer's Disease, Motion Correction
Abnormal accumulation of iron has been found in the subiculum of Alzheimer’s Disease (AD) patients postmortem. This motivated us to push MRI resolution to observe iron accumulation in vivo in AD patients. Using optical prospective motion correction to enable higher resolution at 7T, we imaged 4 healthy controls, 3 mild cognitive impairment, and 1 Alzheimer’s Disease patient with a 60-minute research brain MRI protocol. Hippocampal iron deposits were detected by a neuroradiologist aware of diagnosis in 2 out of 4 MCI/AD subjects despite that mean R2* quantification in the subiculum was not significantly different between healthy control and MCI/AD subjects.Introduction
The hippocampus is a central region of pathology in amnestic Alzheimer’s Disease (AD) and has been the subject of investigation using advanced iron imaging techniques such as QSM [1]. In particular, the subiculum subfield of the hippocampus has been shown to accumulate abnormal iron deposits in postmortem imaging, largely within microglia [2]. Imaging this specific pathological iron deposition in vivo has yet to be achieved, yet it could enable the study of disease progression and present new diagnostic and therapeutic opportunities. While this is an ideal opportunity for 7T MRI, head motion during MRI scans is an enormous obstacle to achieving high-resolution brain images at 7T [3-5]. Motion artifacts corrupt images more easily due to longer scan times, which are required to achieve higher resolution. We have previously demonstrated that our optical prospective motion correction system improves image quality in whole brain QSM and anatomic image sequences [6-8] throughout an entire MRI brain exam [9]. Here, we apply our previous work to: a) acquire our research MRI protocol across a variety of subjects, b) determine whether motion correction-enabled sequences are sensitive enough to detect iron deposits within the subfields of the human hippocampus, in particular in the subiculum of MCI and AD patients.Methods
We conducted 7T MRI using a GE MR950 scanner on 4 health control, 3 mild cognitive impairment (MCI) and 1 Alzheimer’s Disease (AD) subjects who provided written informed consent in accordance with HIPAA. The protocol included: 1) 2D gradient echo (2dfast) (0.1758x0.1758x1.0mm resolution, TR=300ms, TE=20ms, FA=35°, six slices spaced every 6mm) acquired four times to boost SNR, 2) T2-weighted fast spin echo (FSE) (512x512x38, 0.33x0.33x1.5mm, TR=9375ms, TE=50.88ms, cardiac gating on, ARC=2, 8 echoes), 3) T1-weighted BRAVO (256x256x124, 0.94x0.94x1.0mm, TR=9.2ms, TE=3.116ms, FA=10°) for hippocampal subfield segmentation, 4) iron-sensitive QSM sequence, coronal obliqued to achieve high-resolution in-plane coverage of the hippocampus (512x512x58, 0.4297x0.4297x1mm, TR=34.104ms, TE=3.668ms, 5 echoes). The prospective motion correction system and sequences used are described in [7,9]. Briefly, our prospective motion tracking system enables direct line-of-sight from a head-coil-mounted camera to a checkerboard marker placed on the subject’s left temple to record head motion during scanning. The camera is powered by a cable connected to the scanner through the LPCA cover. For image processing, ITK-snap’s distributed segmentation service [10] was used on the FSE and BRAVO sequences to automatically segment hippocampal subfields. R2* maps were computed and registered to FSE to extract mean values from hippocampal subfields. A neuroradiologist with over 12 years of experience who was aware of patient diagnosis visually evaluated each subject’s images.Results
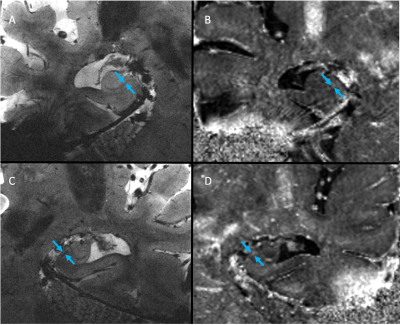
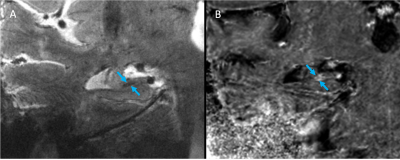
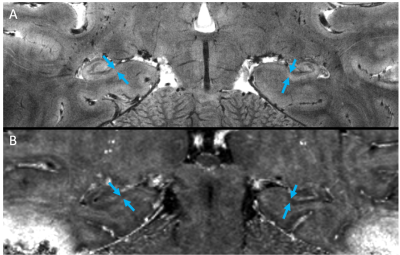
Images were deemed of usable quality in 2/4 cognitively impaired subjects and 2/4 cognitively normal subjects. Images of an MCI subject demonstrated subtle hypointensities in the left and right subiculum (arrows) of the high-resolution GRE image (Fig. 1A,C) and subtle hyperintensities in the same areas of the R2* map (Fig. 1B,D), indicative of iron deposition. In the AD subject, a similar pattern of hypointensities in 2dFAST images and hyperintensities in R2* map was observed, although the spots appeared larger (Fig. 2). Finally, in both healthy control subjects, no GRE hypointensities or R2* hyperintensities were observed in the subiculum (one subject in Fig. 3).Discussion
Motion correction enabled us to achieve high resolution hippocampal images with high enough SNR at 7T for a neuroradiologist to detect abnormal R2* hyperintensities in the subiculum of 2 out of 4 MCI/AD subjects. Microglia containing iron are thought to occur in AD, particularly in the subiculum [4] and R2* has been shown to increase linearly with iron concentration [11]. Thus, we believe this method to be promising for studying localized iron deposition in the subiculum of MCI and AD subjects, especially when the mean R2* value of the entire subfield is not sensitive enough to changes in magnetic susceptibility caused by tiny iron deposits.Conclusion
This is the first demonstration of detecting iron deposits in the hippocampus of human subjects using MRI in vivo. Our 7T motion correction system and high-resolution hippocampal sequences may be useful for detecting image-based biomarkers of disease, such as subicular iron in AD, which current clinical imaging protocols are unable to do. We hope to utilize this technique in a larger cohort of healthy, MCI, and AD subjects to analyze anatomic and QSM changes across hippocampal subfields throughout progression of Alzheimer’s disease.Acknowledgements
The authors would like to acknowledge research support by GE Healthcare, ASNR Boerger Alzheimer’s Fund, and NSF GRFP DGE – 1656518.References
1. Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, Bush AI. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Molecular psychiatry. 2020 Nov;25(11):2932-41.
2. Zeineh MM, Chen Y, Kitzler HH, Hammond R, Vogel H, Rutt BK. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiology of aging. 2015 Sep 1;36(9):2483-500.
3. Mattern H, Sciarra A, Lüsebrink F, Acosta‐Cabronero J, Speck O. Prospective motion correction improves high‐resolution quantitative susceptibility mapping at 7T. Magnetic Resonance in Medicine. 2018.
4. Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction. PLoS One. 2015.
5. Mattern H, Sciarra A, Godenschweger F, Stucht D, LüsebrinkF, Rose G, Speck O. Prospective motion correction enables highest resolution time‐of‐flight angiography at 7T. Magnetic Resonance in Medicine. 2018.
6. DiGiacomo P, Tong E, Maclaren J, Aksoy M, Bammer R, Rutt B, Zeineh M. A novel, coil-integrated camera for prospective optical motion correction of brain imaging at 7T. International Society of Magnetic Resonance in Medicine. 2019, Montreal, Canada.
7. DiGiacomo P, Maclaren J, Aksoy M, Tong E, Carlson M, Lanzman B, Hashmi S, Watkins R, Rosenberg J, Burns B, Skloss TW. A within‐coil optical prospective motion‐correction system for brain imaging at 7T. Magnetic Resonance in Medicine. 2020.
8. DiGiacomo P, Carlson M, Maclaren J, Aksoy M, Wang Y, Spincemaille P, Burns B, Bammer R, Rutt B, Zeineh M. Optical prospective motion correction enables improved quantitative susceptibility mapping (QSM) of the brain at 7T. International Society of Magnetic Resonance in Medicine. 2020, virtual.
9. Carlson M, DiGiacomo P, Burns B, Georgiadis M, Maclaren J, Aksoy M, Rutt B, Zeineh M. Prospective motion correction for a full protocol brain exam in elderly subjects. International Society of Magnetic Resonance in Medicine. 2022, London, United Kingdom.
10. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006 Jul 1;31(3):1116-28.
11. Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005 Aug 15;106(4):1460-5.
Figures