2660
Resting-state global brain activity affects early β-amyloid accumulation and hypoconnectivity in default mode network
Feng Han1, Xufu Liu1, Richard Mailman2, Xuemei Huang2, and Xiao Liu1
1the Pennsylvania State University, State College, PA, United States, 2Pennsylvania State University College of Medicine and Milton S. Hershey Medical Center, Hershey, PA, United States
1the Pennsylvania State University, State College, PA, United States, 2Pennsylvania State University College of Medicine and Milton S. Hershey Medical Center, Hershey, PA, United States
Synopsis
Keywords: Alzheimer's Disease, fMRI (resting state), cerebrospinal fluid
The β-amyloid (Aβ) plaque, an Alzheimer’s disease (AD) hallmark, accumulates first in the default mode network (DMN) regions years before AD diagnosis. The underlying mechanisms remain elusive. Low-frequency (<0.1 Hz) resting-state global brain activity was recently found coupled by cerebrospinal fluid (CSF) movements, and this coupling has been linked to AD pathologies, including Aβ accumulations, presumably due to its role in glymphatic clearance. Here we showed the preferential Aβ accumulation in DMN was related to the reduced engagement of the global brain activity in these regions, partly explained by its failure to reach these regions as cortical propagating waves.Introduction
Over the course of AD progression, early Aβ42 decrease in CSF precedes the cortical Aβ accumulation1, which first accumulates preferentially at the DMN regions and then spreads to lower-order sensory-motor areas2. The mechanisms underlying the specific trajectory remain unclear. High neuronal activity and metabolic stress have been suggested to play a role3–7. The glymphatic system, featured by CSF flow washing out brain wastes, like Aβ, may also contribute to the cortical Aβ pathology and its spreading8–10. Recent studies found the low-frequency (<0.1 Hz) global brain activity assessed by global BOLD (gBOLD) signal was coupled to CSF flow, which can be greatly promoted by drowsy states and sleep, similar to the glymphatic function11,12. The gBOLD-CSF coupling has thus been proposed to reflect global glymphatic function and found associated with various AD pathologies and cognitive impairment in Parkinson’s disease12,13. In fact, the glymphatic clearance, similar to the cortical Aβ accumulation, could be temporally and spatially heterogeneous12,14–16. Interestingly, the resting-state global brain activity shows a striking sensory-dominant pattern17,18, opposite to the early Aβ accumulation at the DMN2, and often takes the form of propagating waves between the DMN and the sensory-motor areas19,20. Altogether, we hypothesize that the spatiotemporal dynamics of the resting-state global brain activity is related to the heterogeneous modulations of glymphatic function and ultimately Aβ accumulation.Methods
We included 144 participants from the Alzheimer's Disease Neuroimaging Initiative project according to the availability of resting-state fMRI (rsfMRI), CSF Aβ42, and 18F-AV45 amyloid PET data. 112 participants were found to have 2-year follow-up (24.0±1.2 months) data of Aβ-PET. Based on the CSF Aβ42 (<192 ng/L as CSF+) and cortical Aβ level (>0.872 SUVR as PET+), all subjects were sub-grouped into three stages (Table 1). Following the previous studies12,13, the CSF and gBOLD signal were extracted and used to derive the gBOLD-CSF coupling, which was further compared among different Aβ stages and correlated with protein markers at cortical brain and CSF across subjects at each stage. For the CSF+/PET- subjects, we computed the 2-year cortical Aβ SUVR changes in all Desikan-Killiany parcels21, and then related this change to the coupling between regional BOLD (rBOLD) and CSF signals quantifying local glymphatic function. The rBOLD was correlated with gBOLD to generate the gBOLD presence at different brain regions, which was further linked to the 2-year cortical Aβ SUVR changes, through their cross-brain contrast between higher-order DMN and lower-order sensory-motor areas. CSF+/PET- subjects with lower CSF Aβ42 have hypoconnectivity at DMN2. Both gBOLD and related dynamic propagation of cortical activation between above higher- and lower-order regions were examined to verify the role of gBOLD in this Aβ pathology. (see details at https://www.biorxiv.org/content/10.1101/2022.07.24.501309v1.full)Results
The global glymphatic function quantified by gBOLD-CSF coupling decreased with advancing Aβ stages (p = 0.044, ordinal regression) and was significantly correlated with various Aβ and tau markers only across CSF+/PET- subjects. (Fig. 1) These subjects had higher 2-year Aβ accumulation at the higher-order DMN and frontoparietal network (FPN) than in lower-order sensory-motor areas, whereas local glymphatic function quantified by the rBOLD-CSF coupling displayed an opposite pattern with weaker (less negative) coupling at the higher-order DMN and FPN. The local glymphatic function was significantly (Spearman’s ρ = 0.48, p = 0.041) correlated, across the CSF+/PET- subjects, with the 2-year Aβ changes in the high-order networks but not the sensory-motor areas (Fig. 2). The preferential 2-year Aβ accumulation at the higher-order regions was further evaluated with a metric of cross-hierarchy contrast (higher-order regions minus the lower-order regions) and found to be associated with the reduced gBOLD presence (correlation between gBOLD and rBOLD) at the higher-order regions, as well as its cross-hierarchy contrast (Fig. 3). We further reproduced the finding of a previous study that the functional connectivity (FC) within the DMN decreased with CSF Aβ42 in the CSF+/PET- subjects2. However, this FC-Aβ association disappeared after recomputing FC with removing gBOLD component, suggesting the role of gBOLD in this association. Moreover, the global propagating waves identified at gBOLD peaks were significantly different between two subgroups of the CSF+/PET- subjects with distinct CSF Aβ42. The sensory/motor to DMN propagations appeared to be weaker, particularly at the higher-order regions, in the subgroup with the lowest CSF Aβ42 group and weaker FC (Fig. 4).Discussions
We linked the spatiotemporal features of the resting-state global brain activity, as well as its coupling to CSF flow, to the spatiotemporal patterns of Aβ accumulation in the earliest preclinical AD. Global glymphatic function was strongly associated with various Aβ and tau markers at a critical disease stage (CSF+/PET-) that features decreased CSF Aβ42 and preferential Aβ accumulation at the higher-order DMN regions. This preferential Aβ accumulation was preceded by reduced local glymphatic clearance that, in turn, was associated with reduced gBOLD presence in these high-order brain regions. The reduced global brain activity contributed to hypoconnectivity, particularly in high-order networks. The disengagement of global brain activity from the DMN may be partly attributed to its failure to reach this higher-order network as propagating waves.Conclusion
Resting-state global brain activity affects Aβ accumulation at a critical preclinical AD stage and in a spatially differentiated way, presumably through its effect on glymphatic clearance.Acknowledgements
This work was supported by the National Institutes of Health (NIH) Brain Initiative award (1RF1MH123247-01), and the NIH R01 award (1R01NS113889-01A1).References
- Jack, C. R. et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
- Palmqvist, S. et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. (2017). doi:10.1038/s41467-017-01150-x
- Buckner, R. L. et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25, 7709–7717 (2005).
- Bero, A. W. et al. Neuronal activity regulates the regional vulnerability to amyloid-β 2 deposition. Nat. Neurosci. (2011). doi:10.1038/nn.2801
- Cirrito, J. R. et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron (2005). doi:10.1016/j.neuron.2005.10.028
- Buckner, R. L. et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. (2009). doi:10.1523/JNEUROSCI.5062-08.2009
- Liang, W. S. et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. U. S. A. 105, 4441–4446 (2008).
- Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science (80-. ). (2013). doi:10.1126/science.1241224
- Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. (2012). doi:10.1126/scitranslmed.3003748
- Tarasoff-Conway, J. M. et al. Clearance systems in the brain - Implications for Alzheimer disease. Nature Reviews Neurology (2015). doi:10.1038/nrneurol.2015.119
- Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science (80-. ). (2019). doi:10.1126/science.aax5440
- Han, F. et al. Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease–related pathology. PLoS Biol. 19, 1–25 (2021).
- Han, F. et al. Decoupling of Global Brain Activity and Cerebrospinal Fluid Flow in Parkinson’s Disease Cognitive Decline. Mov. Disord. 36, 2066–2076 (2021).
- Ringstad, G. et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI insight (2018). doi:10.1172/jci.insight.121537
- Lee, H. et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 (2015).
- Watts, R. et al. Measuring Glymphatic Flow in Man Using Quantitative. Am. J. Neuroradiol. 40, (2019).
- Liu, X. et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 9, 1–10 (2018).
- Li, J. et al. Topography and behavioral relevance of the global signal in the human brain. Sci. Rep. (2019). doi:10.1038/s41598-019-50750-8
- Gu, Y. et al. Brain Activity Fluctuations Propagate as Waves Traversing the Cortical Hierarchy. Cereb. Cortex 31, 3986–4005 (2021).
- Raut, R. V. et al. Global waves synchronize the brain’s functional systems with fluctuating arousal. Sci. Adv. 7, 1–16 (2021).
- Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage (2006). doi:10.1016/j.neuroimage.2006.01.021
Figures
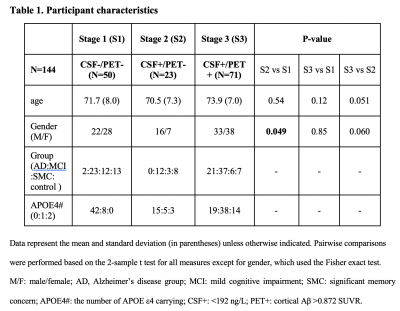
Table 1. Participant characteristics
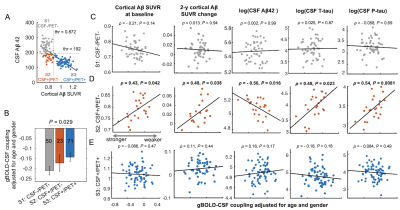
Fig. 1. Stage-dependent associations between various markers of protein aggregation and the global glymphatic function quantified by the gBOLD-CSF coupling. (A) The whole cohort of 144 subjects were sub-grouped into three stages based on the level of CSF Aβ42 and cortical Aβ SUVR. (B) The gBOLD-CSF coupling decreased gradually from S1 to S3 as the Aβ pathology progresses. (C) Only in S2 (CSF+/PET-), the weaker glymphatic function was strongly associated with various Aβ and tau markers.

Fig. 2. Local glymphatic function was related to the Aβ accumulation at the extended DMN among the CSF+/PET- subjects. (A) The 19 CSF+/PET- subjects showed more 2-year Aβ accumulation in the DMN and FPN. (B) Averaged map of the rBOLD-CSF coupling (local glymphatic function) was weaker (less negative) in the DMN regions. (C) The association between the rBOLD-CSF coupling and the 2-year Aβ SUVR change is significant in the higher-order brain networks but not in the lower-order sensory-motor regions.
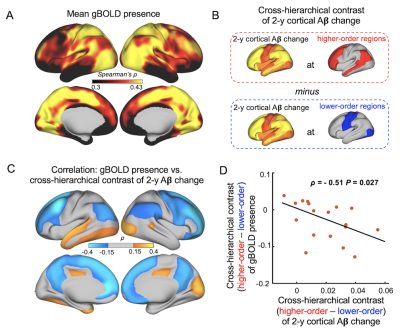
Fig. 3. gBOLD absence at the higher-order regions was related to preferential Aβ accumulation there. (A) Averaged gBOLD presence in the CSF+/PET- subjects was sensory-dominant. (B) Cross-hierarchy contrast quantifies the extent to which the Aβ was preferentially accumulated in the higher-order regions than lower-order. (C-D) gBOLD presence at the higher-order regions, referred to the lower-order, showed strong negative correlations with the cross-hierarchical contrast of 2-year Aβ change.
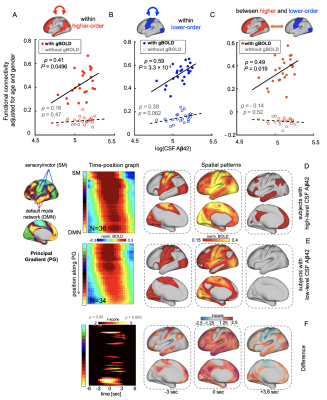
Fig. 4. In the CSF+/PET- subjects, the associations between CSF Aβ42 and the functional connectivity within (A-B) or between (C) the higher- and lower-order regions were diminished by removing the gBOLD component. (D-F) The gBOLD was significantly different as the SM-to-DMN propagating waves in the CSF+/PET- subjects with distinct CSF Aβ42 levels. Compared with higher CSF Aβ42 subjects (N=8), lower level ones (N=8) have weaker SM-to-DMN propagations, and these propagations may fail to reach the DMN.
DOI: https://doi.org/10.58530/2023/2660