2659
Detection of Capillary Leakage in a Transgenic Mouse Model of Alzheimer’s Disease1Boston University, Boston, MA, United States
Synopsis
Keywords: Alzheimer's Disease, DSC & DCE Perfusion
The goal of the project is to explore the possibility of using dynamic contrast-enhanced (DCE)-MRI for detecting subtle blood-brain barrier leakage in a transgenic mouse model of Alzheimer’s disease. The washout slope of Gd concentration was used to assess microvascular integrity. We observed different washout patterns in transgenic relative to control mice. Our findings demonstrated that the washout slope could be used to assess status of blood-brain barrier permeability in AD mice.Introduction
Microvascular leakage has been commonly observed in Alzheimer’s disease (AD), and has been linked to cognitive decline1-4. Recently, dynamic contrast-enhanced (DCE)-MRI has been used to detect subtle brain leakage in human subjects, the results of which suggests blood-brain barrier (BBB) leakage is an early marker of AD, and may proceed significant accumulation of amyloid-beta or hyperphosphorylated-tau, two classic hallmarks of AD pathology3. Transgenic mouse models are valuable tools for tackling underlying mechanisms of BBB leakage in AD progression. However, the application of DCE-MRI in the preclinical setting may face some technical hurdles as small vessel sizes introduce large partial volume effects and lead to inaccurate assessment of vascular input function, which may compromise the traditional data analysis using Tofts or Patlak models5. To address this issue, we explored the possibility of using the slope of the washout curve as a permeability marker to detect subtle BBB leakage in AD mouse brains.Methods
MRI data were acquired in vivo on a mutant tau transgenic mouse model of AD, P301S (PS19), using a 9.4T scanner (Bruker BioSpec 94/20). During the MRI experiment, mice were anesthetized (1–2% isoflurane, oxygen flow rate: 1-2L/min) and their respiration was monitored. Various flip angles (VFA) MRI was performed prior to the contrast administration. Key parameters were: repetition time (TR) = 150ms, echo time (TE) = 1. 9ms, field of view (FOV) = 20mm2, slice thickness = 0.7mm, gap = 0.1mm, matrix = 200x200, slice number = 9, number of averages = 2, flip angles = 8°, 11°, 14°, 18°, 22°, 26°, and 30°. Key parameters for DCE-MRI were: TR = 56.13ms, TE = 1.52ms, FOV = 20mm2, slice thickness = 0.7mm, gap = 0.1mm, matrix = 200x200, slice number = 9, flip angle = 25°, repetition = 160. Ten baseline scans were obtained pre-injection, and 150 scans were acquired post-injection. The contrast agent administered is Gadobutrol (1mmol/ml, Bayer, Canada). Gadobutrol was diluted 20 times (in saline) and injected via the tail vein using a small animal power injector (model PHD2000, Harvard Apparatus, MA) with an injection volume of 100ml, and an injection speed of 15ml /s. T1 mapping obtained from VFA MRI was used to transform variations in DCE-MRI intensity to changes of Gd concentration. The washout slope was calculated from the time of peak concentration appeared till the end of acquisition window. The obtained slope was used as a surrogate for the BBB permeability. T1-weighted (T1W) images were acquired post Gd injection using the fast low-angle shot (FLASH) sequence for identifying anatomic structures. Key parameters were: TR=344ms, TE=3ms, FOV=20mm2, slice thickness=0.4mm, matrix=384x384, slice number=54, averages=3, FA=30°. Data from P301S mice at 12 months old were compared to age and gender matched non-transgenic littermates.Results
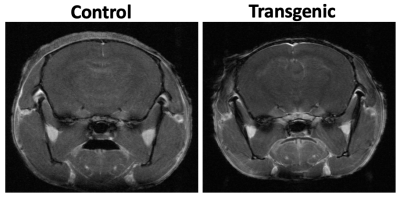
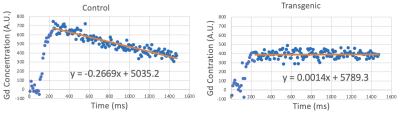
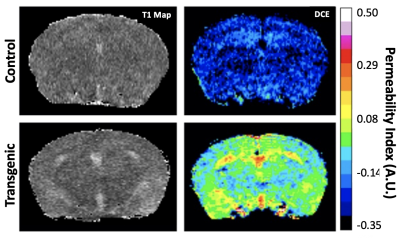
The representative T1W images of both transgenic (P301S) and non-transgenic control mice are shown in Figure 1. Manual ROIs were drawn on the hippocampus and the average Gd concentration within the ROI before and after Gd injection are shown in Figure 2. The washout slopes in the hippocampus are -0.2669 a.u./ms and 0.0014 a.u./ms for the non-transgenic control and transgenic mice, respectively. At 12 months of age, BBB permeability in the representative P301S mouse was increased globally across the brain compared to the non-transgenic control (Figure. 3). The increase of permeability of the P301S mouse was most prominent in the hippocampus, and followed by the entorhinal and cingulate cortices.Discussion
Our results demonstrated that the washout slope of Gd showed a dramatic difference between AD and non-transgenic control mice. The non-transgenic control mouse showed negative washout slope, indicating Gd clearance. On the other hand, the increased possibility of BBB leakage in the transgenic mouse is accompanied by a flat washout slope, indicating retained Gd in the tissue and impaired Gd clearance.Conclusion
We piloted the DCE-MRI in detecting subtle BBB leakage in a transgenic mouse of AD, and demonstrated that the washout slope of Gd concentration could be used as a permeability marker to assess subtle BBB leakage in AD mice.Acknowledgements
No acknowledgement found.References
1. van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Hofman PA, Verhey FR, Backes WH. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 2016;281(2):527-35. Epub 20160531. doi: 10.1148/radiol.2016152244. PubMed PMID: 27243267.
2. Lecler A, Fournier L, Diard-Detoeuf C, Balvay D. Blood-Brain Barrier Leakage in Early Alzheimer Disease. Radiology. 2017;282(3):923-5. doi: 10.1148/radiol.2017162578. PubMed PMID: 28218884.
3. Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270-6. Epub 20190114. doi: 10.1038/s41591-018-0297-y. PubMed PMID: 30643288; PMCID: PMC6367058.
4. Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D’Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71-6. doi: 10.1038/s41586-020-2247-3.
5. Sourbron SP, Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR in Biomedicine. 2013;26(8):1004-27. doi: 10.1002/nbm.2940.
Figures