2652
Effects of aquaporin-4 on neurovascular coupling and brain network in Alzheimer’s disease mice model1The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China
Synopsis
Keywords: Alzheimer's Disease, fMRI, neurovascular coupling
Neurovascular uncoupling can be found in the early stage of AD continuum. The effect of AQP4 content on neurovascular coupling (NVC) in AD mice is unclear. Adult 5xFAD mice and wild-type mice were employed in this study. Mice received TGN-020, an AQP4 inhibitor, intraperitoneally. The results showed that 20 min after the injection of TGN-020, reduced ReHo in the basal area of the frontal lobe, a trend of reduced functional connectivities and disordered network properties were found among the AQP4-inhibited WT and 5xFAD mice. Therefore, Inhibition of AQP4 may reduce the NVC, and disorder the functional network.Introduction
By far no effective treatment has been found for Alzheimer's disease (AD). As neurovascular uncoupling, which leads to increased Aβ production and decreased Aβ clearance, plays an initial role in promoting AD pathologies, it could be regarded as an early pathological predictor for AD1. Aquaporin-4 (AQP4), a functional protein on astrocytes, may affect neurovascular coupling (NVC) by regulating astrocytic function2. Neurovascular coupling (NVC) is the basis of blood oxygen level dependent (BOLD) fMRI3. The aim of this study is to investigate the effect of AQP4 content on NVC in AD mice.Method
Adult (12 weeks) 5xFAD mice and wild-type mice of both sexes were employed in this study. Anesthesia was induced with 4% isoflurane and an injection of 0.05 mg/kg medetomidine. Mice received TGN-020 (250mg/kg), an AQP4 inhibitor, intraperitoneally.Images were acquired on a 9.4T Bruker MR system (BioSpec 94/20 USR, Bruker), using an 86-mm volume transit RF coil and a single channel surface head coil. Anesthesia was induced with 4% isoflurane and an injection of 0.05 mg/kg medetomidine, and sedation level was maintained by continuous infusion of medetomidine and 0.5% isoflurane. Tooth bar and ear bars were used to restrain mice on an MRI-compatible cradle for image acquisition. Functional images were acquired using the gradient-echo echo planar imaging (GE-EPI) sequence with the following parameters: repetition time (TR): 1000 ms, echo time (TE): 15 ms, field of view (FOV): 32 mm × 12 mm, matrix: 160 × 60, 30 interlaced slices of 0.4 mm slice thickness, and a total acquisition time of 5 min.
Preprocessing steps of the rsfMRI images, such as realign, slice timing, normalization and smooth, and voxel-wised group comparisons were performed by the spmratIHEP4 toolbox of spm12. Amplitude Low Frequency Fluctuations (ALFF) and regional homogeneity (ReHo) were calculated by the RESTplus (V1.22) toolbox. The Turone Mouse Brain Template and Atlas (TMBTA) (fig.1) was used for functional network construction, and network properties and group-wise comparisons were performed by the GRETNA (V2.0.0) toolbox.
Results
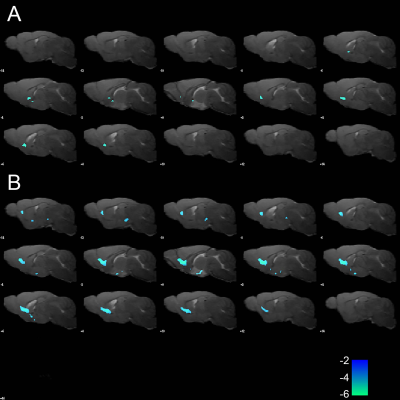
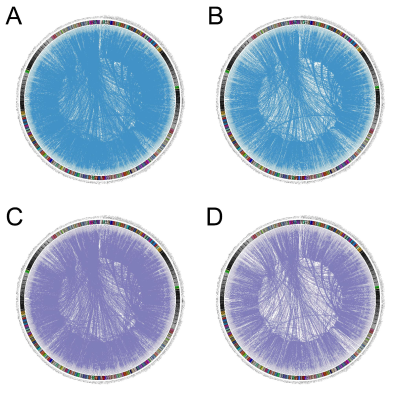
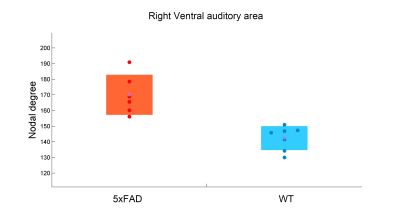
20 min after the injection of TGN-020, reduced ReHo was found in the basal area of the frontal lobe (fig.2) in both WT (n=7, p < 0.005) and 5xFAD mice (n=6, p < 0.001), compared with non-inhibited WT (n=12) and 5xFAD (n=10) mice, respectively. No significant differences in ReHo were found between the WT and 5xFAD mice, with and without AQP4 inhibitor. No significant differences in ALFF were found in any contrast of group-wise comparisons.For functional network analysis, however, a trend of reduced functional connectivity was observed in both WT and 5xFAD mice after TGN-020 injection, compared with their non-inhibited counterparts (fig.3). While no significant differences of global and nodal topological properties were found between the WT and 5xFAD mice, a trend of disordered network properties, such as nodal degree, nodal efficiency, and shortest path were found among the AQP4 inhibited WT and 5xFAD mice. A reduction of nodal degree was observed in AQP4-inhibited WT mice (fig.4), in comparison with AQP4-inhibited 5xFAD mice. However, no group-wise topological differences remain significant after FDR corrections.
Discussion
In this study, we demonstrated the resting-state functional alterations mediated by TGN-020, an AQP4 inhibitor, in WT and 5xFAD mice. Our findings suggest that while reduced regional brain activities were found in AQP4-inhibited 5xFAD mice, network-level dysfunctions were found in both WT and 5xFAD mice after AQP4 inhibition. A trend of decreased nodal degree and nodal efficiency was observed mainly in AQP4-inhibited WT mice, in comparison with AQP4-inhibited 5xFAD mice.The alteration of the BOLD signal in WT and 5xFAD mice after the injection of TGN-020 demonstrated that the reduction of AQP4 may affect NVC. Furthermore, recent studies have shown that astrocyte calcium dysfunction causes early network hyperactivity in AD, and recovery of astrocytes can improve brain areas connectivity and cognitive function5. AQP4 is localized to the astrocyte end foot and its change may affect the function of astrocytes. Another study found that AQP4 is involved in retinal neurovascular dysfunction and can be used to treat diseases by regulating AQP4 to improve neurovascular function6. The pieces of evidence suggest the potential value of AQP4 as a therapeutic target for AD.
One of the limitations of this study is anesthesia, which would result in sparse functional connectivity of the brain and unpredictable results. Secondly, our findings would benefit from a larger sample size, especially with the AQP4-inhibited WT and 5xFAD mice.
Conclusion
Inhibition of AQP4 may reduce the NVC and disorder the functional network. 5xFAD mice were more sensitive to AQP4 inhibitors, which suggested that decreased AQP4 may play a role in reducing NVC in the AD continuum.Acknowledgements
No acknowledgement found.References
1. Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease[J]. Nature reviews Neuroscience. 2017,18(7):419-34.
2. Jukkola P, Gu C. Regulation of neurovascular coupling in autoimmunity to water and ion channels[J]. Autoimmunity reviews. 2015,14(3):258-67.
3. Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, et al. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences[J]. Nature communications. 2021,12(1):3190.
4. Binbin N, Di W, Shengxiang L, et al. A stereotaxic MRI template set of mouse brain with fine sub-anatomical delineations: Application to MEMRI studies of 5xFAD mice. Magn Reson Imaging. 2019; 57: 83-94.
5. Shah D, Gsell W, Wahis J, Luckett ES, Jamoulle T, Vermaercke B, Preman P, Moechars D, Hendrickx V, Jaspers T, Craessaerts K, Horré K, Wolfs L, Fiers M, Holt M, Thal DR, Callaerts-Vegh Z, D'Hooge R, Vandenberghe R, Himmelreich U, Bonin V, De Strooper B. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer's disease[J]. Cell Rep. 2022 Aug 23;40(8):111280.
Figures