2650
Metabolic Imaging using Chemical Exchange Saturation Transfer in Rat Transgenic Model of Alzheimer’s Disease Comorbid with Metabolic Syndrome
Dustin Loren Velasco Almanza1,2, Wilfred Lam2, Margaret Koletar2, Mary Hill3, JoAnne McLaurin3,4, Greg Stanisz1,2, and Bojana Stefanovic1,2
1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 3Biological Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 4Laboratory Medicine and Pathology, University of Toronto, Toronto, ON, Canada
1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 3Biological Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 4Laboratory Medicine and Pathology, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Alzheimer's Disease, CEST & MT
Our study characterized the hippocampal glucose uptake in an Alzheimer’s Disease rat model (TgF344AD) comorbid with metabolic syndrome modeled by cafeteria (CAF) diet. Glucose uptake was evaluated by detecting 2-deoxy-D-glucose (2DG) using chemical exchange saturation transfer (CEST) MRI in 6- and 12-month-old cohorts, reflecting pre-clinical and established disease stages, respectively. CAF diet attenuated glucose uptake of both non-transgenic and transgenic-AD at 6 months of age; whereas at 12 months of age, CAF diet enhanced the hippocampal glucose uptake of TgAD but attenuated it in chow-fed TgAD, when compared to that in correspondingly fed nTg littermates.Introduction
By the time a patient is diagnosed with Alzheimer’s disease (AD), neuronal loss has already occurred, yet intervening before significant atrophy is thought key for abating cognitive decline. There is thus a pressing need for the identification of sensitive and specific biomarkers of initial AD pathology to enable earlier diagnosis. Disruption of brain glucose metabolism has been observed in AD-susceptible brain regions (most prominently hippocampus)1,2 before the onset of significant cognitive symptoms in patients, making glucose metabolism impairment of interest for early detection of AD and a potential target of early interventions. 2-deoxy-D-glucose (2DG) is a labeled glucose that gets taken up by cells via glucose transporters yet remains trapped for hours intracellularly3. 2DG is detected using chemical exchange saturation transfer (CEST) MRI, a highly sensitive technique that can detect 2DG at low concentrations (µM - nM)4. This project utilized the TgF344-AD rat model, exhibiting amyloidogenesis, tau pathology, frank neuronal loss, and progressive cognitive decline5. Since the translation of preclinical findings has been long confounded by the lack of incorporation of prominent AD comorbidities, we have presently incorporated a model of metabolic syndrome in the TgF344-AD rats. Cafeteria (CAF) diet comprises grocery store-purchased food items with variations of nutrients, energy-dense, and highly palatable food to animals that imitate obesogenic features of the human diet6. TgF344-AD rats and their non-transgenic littermates were subjected to CAF diet to model human metabolic syndrome and their hippocampal glucose uptake was investigated using 2DG CEST MRI.Method
TgF344-AD rats and their non-transgenic (nTg) littermates were subjected to CAF diet for 3 months prior to imaging. The rats were imaged at 6 and 12 months of age, representing early amyloid and established amyloid, tau pathology, and neuronal loss stages, respectively. Animals were anesthetized using isoflurane delivered via nose cone and scanned on a 7T MRI scanner BioSpec 70/30 USR with BGA-12SHP gradients running ParaVision 6.0.1, Bruker BioSpin, Billerica, MA, USA). The saturation transfer-weighted images were acquired using one 490-ms block RF saturation pulse with amplitude B1 = 1.5µT per k-space line followed by a single-slice FLASH acquisition (TR/TE = 500/3 ms; flip angle = 30°; FOV = 20 × 20 mm2; in-plane resolution = 0.31 × 0.31 mm2, slice thickness = 1.5 mm)7,8. Dummy scan, reference scan (at 667 ppm frequency offset to avoid saturation transfer effects), 12 repetitions of three-offset acquisition (at 1.2, 2.0, 2.9 ppm reflecting OH at third, fourth, and first carbon in 2DG), and another reference scan compromised the 20.7 min dynamic acquisition segment9. One such segment was acquired during baseline and three more were acquired following the onset of 2DG injection. The mean magnetization transfer ratio (MTR; defined as 1 – S/S0 where S and S0 are the reduced water signal and the signal without saturation respectively) of the baseline segment was subtracted to produce the MTR change (ΔMTR) time course7. To quantify the uptake of 2DG, the mean ΔMTR between the onset and offset times was calculated where onset and offset times for the time series were defined for each frequency offset as the time when the ΔMTR rose and fell to half of its maximum level respectively. Two-way unbalanced ANOVA with genotype and sex as the main effects was used to evaluate the differences in glucose uptake.Results
At 6 months of age, there was no significant interaction between diet and genotype. However, diet had a significant effect on the mean ΔMTR (at 2.9ppm: 24% of the variation, p = 0.0312) indicating that the CAF diet decreases the hippocampal glucose uptake in both 6-month-old nTg and TgAD rats. There was no significant difference in glucose uptake between nTg and TgAD rats exposed to either diet. In contrast, at 12 months of age, the interaction of diet and genotype was significant (at 2.9ppm: 17% of the variation, p = 0.0487) indicating that the CAF diet affects the hippocampal glucose uptake of nTg vs. TgAD rats differently. While chow-fed TgAD rats showed lower ΔMTR compared to chow-fed nTg (at 2.9ppm: difference of -0.1275 ± 0.4163, p = 0.7672), CAF-fed TgAD rats showed significantly higher mean ΔMTR compared to CAF-fed nTg rats (at 2.9ppm: difference of 0.9928 ± 0.2840, p = 0.0058). The higher glucose uptake in CAF-fed TgAD rats suggests beneficial effects of the CAF diet in the early stages of AD pathology.Conclusion
We postulate that at the pre-clinical stage (6 months of age), CAF diet is detrimental to both non-transgenic and AD rats as demonstrated by attenuated glucose uptake. However, the high caloric intake afforded by CAF diet exposure may be beneficial in AD-challenged rats (12 months of age) due to their incipient mitochondrial dysfunction and hence decreased brain metabolic efficiency. The contrast between CAF- and chow-fed TgAD rats’ metabolism supports diet as a modifiable risk factor for AD.Acknowledgements
This study had funding support from Canadian Consortium on Neurodegeneration in Aging (CCNA, Canada; Grant ID: CAN 163902); Canadian Institutes of Health Research (CIHR, Canada; Grant ID: PJT173409) and National Institutes of Health (NIH, USA; Grant ID: 1R01AG057665- 01A1).References
1. Mosconi L, De Santi S, Brys M, et al. Hypometabolism and Altered Cerebrospinal Fluid Markers in Normal Apolipoprotein E E4 Carriers with Subjective Memory Complaints. Biol Psychiatry. 2008;63(6):609-618.2. Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676-692.
3. Jin T, Mehrens H, Wang P, Kim SG. Glucose metabolism-weighted imaging with chemical exchange-sensitive MRI of 2-deoxyglucose (2DG) in brain: Sensitivity and biological sources. NeuroImage. 2016;143:82-90. doi:10.1016/j.neuroimage.2016.08.040
4. Nasrallah FA, Pagès G, Kuchel PW, Golay X, Chuang KH. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab. 2013;33(8):1270-1278.
5. Cohen RM, Rezai-Zadeh K, Weitz TM, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J Neurosci. 2013;33(15):6245-6256.
6. Gomez-Smith M, Karthikeyan S, Jeffers MS, et al. A physiological characterization of the Cafeteria diet model of metabolic syndrome in the rat. Physiol Behav. 2016;167:382-391.
7. Lam WW, Oakden W, Murray L, et al. Differentiation of Normal and Radioresistant Prostate Cancer Xenografts Using Magnetization Transfer-Prepared MRI. Sci Rep. 2018;8(1):10447.
8. Tan Z, Lam WW, Oakden W, et al. Saturation transfer properties of tumour xenografts derived from prostate cancer cell lines 22Rv1 and DU145. Sci Rep. 2020;10(1):21315.
9. Joo IL, Lam WW, Oakden W, et al. Early alterations in brain glucose metabolism and vascular function in a transgenic rat model of Alzheimer’s disease. Progress in Neurobiology. 2022;217:102327. doi:10.1016/j.pneurobio.2022.102327
Figures

Figure 1. Dynamic CEST ΔMTR time courses. CEST MRI can detect the uptake of 2DG in the hippocampus by probing specific frequency offsets (1.2 ppm ~ OH at the third and fourth carbon in 2DG, 2.0 ppm ~ OH at the first carbon in 2DG). Change in the signal at these offsets reflects changes in 2DG uptake. (A-C) The mean and SEM of the ΔMTR in the hippocampus of rats fed with standard CHOW diet vs. CAF diet at each frequency offset were interrogated. The time origin is the start of the 6-min 2DG infusion, which is indicated by the gray-shaded rectangle.
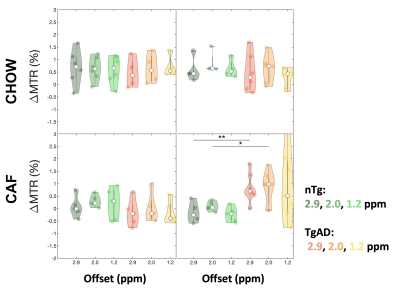
Figure 2. Mean ΔMTR violin plots. Violin plots of the mean ΔMTR at each frequency offset between onset and offset times. Onset and offset times for the time series were defined for each frequency offset as the time when the ΔMTR rose and fell to half of its maximum level respectively. At 12 months, the onset and offset times were derived from the TgAD group with clear wash-in and wash-out signal behavior and were also applied to the nTg group. *p < 0.05 and **p < 0.01.
DOI: https://doi.org/10.58530/2023/2650