2646
Central Thalamic Intermittent Theta-Burst Stimulation Ameliorated Memory Impairment in Alzheimer's Disease Mouse Model1Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Taipei, Taiwan, 2PhD Program in Medical Neuroscience, Taipei Medical University, Taipei, Taiwan, 3Abbott Neuromodulation, Austin, TX, United States, 4Department of Neurology, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, 5Department of Neurology, Tzu Chi University, Hualien, Taiwan
Synopsis
Keywords: Alzheimer's Disease, fMRI, Intermittent theta-burst stimulation (iTBS)
Intermittent theta-burst stimulation (iTBS) has been hypothesized to be a more effective paradigm for deep brain stimulation (DBS) in treating various neurological disorders by altering neuronal circuits. However, the effect of iTBS in improving memory deficits in AD patients remains unknown. Combining iTBS in central thalamus (CT-iTBS) with functional magnetic resonance imaging, our results demonstrated the restoration of brain functional connectivity (FC) in corticolimbic circuit of AD mice model, accompanied with enhancing memory cognitive function in novel object recognition test and T-maze test. These results revealed that CT-iTBS could be an effective treatment for improving symptoms of individuals with AD.
Introduction
Alzheimer’s disease (AD), as the most common cause of dementia 1, 2, has yet to develop a present drug capable of preventing the underlying progressing neurodegeneration 3. Several non-pharmaceutical approaches are currently being tested, including deep brain stimulation (DBS). As a neuromodulation therapy, DBS has been proven to be viable for various neurological disorders 4, by enhancing or interrupting different connections within the brain 5.Recently, a different stimulation paradigm, intermittent theta-burst stimulation (iTBS) has been hypothesized to alter cognitive functions in a more efficacious way 6. By delivering in rhythmic bouts of frequency at 3-8 Hz, iTBS recapitulates natural brain rhythm to immediately provoke theta oscillations in the brain, which have profound functional relevance to cognition 7. The efficacy of iTBS in improving memory deficits in AD patients, is yet to be found. Various brain structures have been considered as the target for enhancing cognitive functions, including central thalamus (CT) 8, a signal hub in the brain, might be a promising target for DBS therapy in AD patients. In this study, we used functional magnetic resonance imaging (fMRI) to determine the efficacy of iTBS for altering brain activation, then validated the therapeutic effect of CT-iTBS in the triple-transgenic AD model (3×TgAD), using behavioral tests and fMRI to evaluate the potential of CT-iDBS treatment.
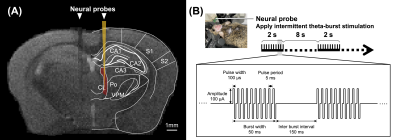
Methods
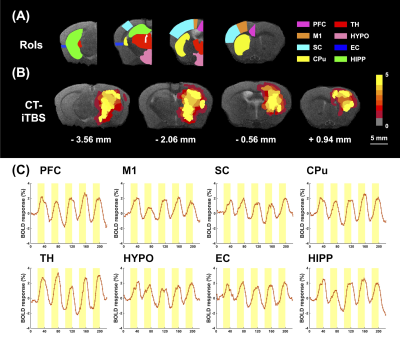
In this study, five adult C57BL/6 mice were used for identifying the activated brain regions during unilateral CT-iTBS under MRI scanning. Then, twenty 8-month-old 3×TgAD mice as disease model and ten age-match C57BL/6 mice as healthy control were used to investigate the therapeutic effect of CT-iTBS by resting state fMRI (rsfMRI) and behavioral tests, all thirty mice were separated into three groups: without iTBS (AD-Sham) group (N = 10), with iTBS (AD-iTBS) group (N = 10), and healthy control (HC) group (N = 10). All mice received implantation surgery with two MRI-compatible neural probes into the bilateral CT (AP: − 1.56 mm, ML: ± 0.7 mm, DV: − 3.0 mm). After one week recovery, mice received 7 days of bilateral iTBS (or sham iTBS) for thirty minutes per day. Biphasic electrical current of 100µA with a pulse width of 100µs and burst width of 50 ms at 5 Hz burst rate and 200 Hz intra-burst rate (10 pulse per bust) was delivered (Figure 1). Novel object recognition test (NOR) and T-maze test were used to assess long-term recognition memory and working memory. The preference index (PI) of NOR was calculated as memory performance. Mice in T-maze tends to choose the arm not visited before, indicating spontaneous alternation. MRI images were acquired from a 7 Tesla Bruker MRI system. fMRI data were acquired using gradient-echo planar imaging sequence with the following parameters: repetition time = 2,000 ms, echo time = 20 ms, 14 coronal slices, slice thickness = 0.5 mm, field of view = 20 × 20 mm2. matrix size = 80 × 80. The regions of interest (ROIs), including prefrontal cortex (PFC), somatosensory cortices (SC), motor cortex (M1), caudate putamen (CPu), hippocampus (HIPP), hypothalamus (HYPO), thalamus (TH), and entorhinal cortex (EC). Functional connectivity (FC) was analyzed with significant results using a one sample t test (thresholds: p < 0.05 and cluster size > 200 voxels) by the FMRIB Software Library v6.0 and the Analysis of Functional NeuroImages software. Significant differences among multiple groups were obtained by Kruskal–Wallis test followed by Dunn’s multiple comparisons test. Significance was inferred at p-value < 0.05. Data were presented as the mean ± standard error of the mean.Results
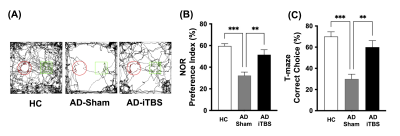
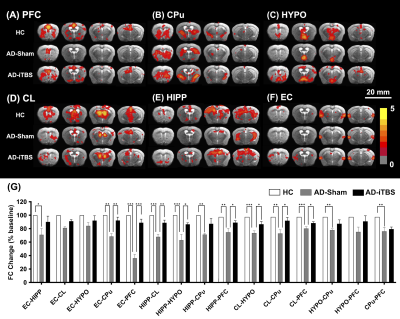
CT-iTBS was found to activate a variety of cortical and subcortical areas, eliciting robust responses that were also highly synchronized with the stimulation paradigm (Figure 2). Applying CT-iTBS as treatment for AD mouse model, we observed that AD-iTBS group showed significant higher PI in the NOR test as well as significant higher correct choice rate for T-maze test when compared to AD-sham group, indicating the restoration of memory deficit in AD mice (Figure 3). The rsfMRI results demonstrated significantly higher FC between the PFC, Hip, EC, and CPu after CT-iTBS treatment, which suggesting the enhancement of corticolimbic circuit and contributing to the improvement of memory function (Figure 4).Discussion
The widespread and strong brain response activated by CT-iTBS may due to its recapitulating natural brain rhythm like pattern to provoke theta oscillations in the brain 9 , which was functionally related to cognition 10, 11. The broad region activated by CT-iTBS at cortical (ACC and M1) and subcortical (CPu, Hip, CL, and HypoTH) were associated with corticolimbic, corticostriatal, and thalamocortical networks, which were related to modulate skill learning with reward behavior, consciousness, sleep/wake cycle, and memory cognitive function 12, 13. After applying CT-iTBS in AD mice model, the increase of FC strength in the above brain networks indicated the memory cognitive function enhancement, which was also consistent with behavioral test results.Conclusion
In conclusion, iTBS has been proven to elicit robust and widespread brain activation responses. By applying CT-iTBS to AD mice model, the restoration of FC in the corticolimbic circuit and the enhancement of memory cognitive function were found, suggesting CT-iTBS would be an effective treatment for improving the cognitive function of individuals with AD.Acknowledgements
This work is financially supported by National Science and Technology Council under Contract numbers of MOST 111-2321-B-A49-005-, 111-2314-B-303-026-, 111-2221-E-A49-049-MY2, and 111-2314-B-038-059-MY3.
References
1. Breijyeh Z, and Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020; 25(24): 5789.
2. Qiu C, Kivipelto M, and Von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues in clinical neuroscience. 2022; 11(2): 111-128.
3. Yiannopoulou KG, Anastasiou AI, Zachariou V, et al. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019; 7(4): 97.
4. Johnson MD, Lim HH, Netoff TI, et al. Neuromodulation for brain disorders: challenges and opportunities. IEEE Transactions on Biomedical Engineering. 2013; 60(3): 610-624.
5. Lv Q, Du A, Wei W, et al. Deep brain stimulation: a potential treatment for dementia in Alzheimer's disease (AD) and Parkinson's disease dementia (PDD). Frontiers in neuroscience. 2018; 12:360.
6. Horn MA, Gulberti A, Gülke E, et al. A new stimulation mode for deep brain stimulation in Parkinson's disease: Theta burst stimulation. Movement Disorders. 2020; 35(8): 1471-1475.
7. Solomon EA, Sperling MR, Sharan AD, et al. Theta-burst stimulation entrains frequency-specific oscillatory responses. Brain Stimulation. 2021; 14(5): 1271-1284.
8. Li S-J, Lo Y-C, Lai H-Y, et al. Uncovering the modulatory interactions of brain networks in cognition with central thalamic deep brain stimulation using functional magnetic resonance imaging. Neuroscience. 2020; 440: 65-84.
9. Wang C-F, Yang S-H, Lin S-H, et al. A proof-of-principle simulation for closed-loop control based on preexisting experimental thalamic DBS-enhanced instrumental learning. Brain Stimulation. 2017; 10(3): 672-683.
10. Herrero JL, Smith A, Mishra A, et al. Inducing neuroplasticity through intracranial θ-burst stimulation in the human sensorimotor cortex. Journal of Neurophysiology. 2021; 126(5): 1723-1739.
11. Demeter E. Enhancing cognition with theta burst stimulation. Current Behavioral Neuroscience Reports. 2016; 3(2): 87-94.
12. James GA, Kearney-Ramos TE, Young JA, et al. Functional independence in resting-state connectivity facilitates higher-order cognition. Brain and cognition. 2016; 105: 78-87.
13. Urban KR, Layfield DM, and Griffin AL. Transient inactivation of the medial prefrontal cortex impairs performance on a working memory-dependent conditional discrimination task. Behavioral neuroscience. 2014; 128(6): 639.
Figures