2644
Quantification of myelination in Children with Attention-deficit/hyperactivity Disorder: A Comparative Assessment with Synthetic MRI and DTI
liping lin1, Yingqian chen1, Yan Dai1, yan zi1, Mengsha zou1, Long qian2, Meina Liu3, Hongyu zhang3, Zhiyun yang1, and Shu su1
1Department of Radiology, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, Guangzhou, China, 2Department of Biomedical Engineering, College of Engineering, Peking University, Beijing, Beijing, China, 3Department of Pediatric, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, Guangzhou, China
1Department of Radiology, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, Guangzhou, China, 2Department of Biomedical Engineering, College of Engineering, Peking University, Beijing, Beijing, China, 3Department of Pediatric, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, Guangzhou, China
Synopsis
Keywords: White Matter, White Matter, attention-deficit/hyperactivity disorder, Synthetic MRI, myelin volume fraction, myelin volume, diffusion tensor imaging, Children.
Evaluation of myelin content is crucial for attention-deficit/hyperactivity disorder (ADHD) and other neurodevelopmental disorders. Diffusion tensor imaging (DTI) is a usual tool to assess white matter structural change in ADHD but it’s indirectly. Synthetic MRI–based (SyMRI-based) method, as a suitable quantitative technique, can investigate myelin content through quantifying whole-brain myelin volume fraction (MVF) and myelin volume (MYV). We aim to evaluate myelin estimation using SyMRI–based method and compared it with established DTI metrics in ADHD children.Introduction/Purpose:
Attention-deficit/hyperactivity
disorder (ADHD) is one of the most common neuro-developmental disorders, with
core symptoms of inattention and impulsivity/hyperactivity (1),
and may persist into adolescence and adulthood. Hence,
understanding the pathophysiological mechanism in ADHD remains vital. Nowadays,
diffusion tensor imaging (DTI) is a usual tool to assess WM structural change
in ADHD through measuring restricted diffusion of water molecules in tissue (2). Nevertheless, the DTI-derived metrics
indirectly explored the axonal and myelin integrity (2). Besides, ADHD-related DTI findings
were inconsistent (3). Therefore,
evaluation of specific WM microstructure alteration (e.g., myelin) may provide
a supplementary point for understanding the pathophysiological mechanism in
ADHD. Myelin content can be evaluated by T1w/T2w ratio, myelin water imaging,
magnetization transfer ratio, synthetic magnetic resonance imaging (SyMRI) (4),
and et al. Among them, SyMRI based on a rapid, simultaneous quantification of
relaxation times, and proton density from a single MRI quantification scan (4).
In our previous SyMRI-based study, compared to typically developing
(TD) children, pediatric ADHD demonstrated no significant
differences in whole-brain myelin content (5),
but the regional myelin alterations were still unknown and intriguing.
Recently, Roh-Eul et al. put forward a valuable method that regional myelin
estimation combining automatic segmentation of the whole brain based on 3D
T1-weighted images, which can be performed individually (6).
Thus, the aims of this study were 3-fold: firstly, to identify brain myelin
changes at a global and regional level between ADHD and TDs, and secondly, to
compare the congruency of the novel SyMRI–based myelin estimation with
established DTI-derived parameters. Thirdly, to evaluate the relationship between
significant myelin content alterations and symptom severity level of ADHD.
Methods
A total of 53 ADHD
and 48 age-, gender-, and handedness-matched TD children were recruited.
Compared with TDs, global and regional myelin content (myelin volume fraction
[MVF], myelin volume [MYV]) were assessed with analysis of covariance (ANCOVA).
Besides, regional diffusion metrics (fractional anisotropy and
mean/radial/axial diffusivity) were also evaluated with ANCOVA. Furthermore,
the relationship between significant MRI parameters and clinical symptom
severity were assessed using the partial correlation analysis in ADHD.
Results
There were no
between-group differences of whole-brain myelin content. Compared to TDs,
atlas-based regional analysis revealed higher mean MVF at widespread regions,
which mostly located in bilateral internal capsule, external capsule, corona
radiata, and corpus callosum, as well as in left tapetum, left superior
fronto-occipital fascicular, and right cingulum (all PFDR-corrected
< 0.05) in ADHD (Figure1). Increased MYV were found in most of
above-mentioned regions (Figure1). Abnormalities of DTI
metrics were mainly at the bilateral corticospinal tract and medial lemniscus.
Besides, MYV at right posterior limb of internal capsule (r = 0.377, P = 0.040) and left superior
corona radiata (r = 0.375, P = 0.041) were positively correlated with
cancellation test scores in ADHD (Figure2).
Discussion
In this study, we
evaluated a new SyMRI–based myelin estimation and compared it with established
DTI metrics in ADHD. The main findings were as follows: 1) increased regional
MVF and MYV rather than whole-brain were found in ADHD using SyMRI method; 2)
the changes of DTI metrics were mainly at the bilateral CST and ML; 3) the
regional MYV in right RLIC and left SCR was positively associate with the
clinical symptoms of ADHD.
No
significant differences in global WMV, MYV, and MVF between ADHD and TDs were
observed, which were occurrent with our previous study (5).
In current study, ADHD showed increased regional myelin content other than
whole-brain at the crucial pathway, which mainly located in the frontostriatal
tract, posterior thalamic radiation, and corpus callosum. To
be specific: 1) The frontostriatal tract (involving
PLIC, ALIC, CG, ACR, SCR, and EC) is a key component of the reward processing
circuitry and in ADHD, a number of studies have reported altered white matter
microstructure of this tract (7, 8).
2) The deficits in the early visual information processing and decreased
small-world network metrics including nodal efficiency in multiple brain
regions involving visual network were reported in pediatric ADHD (9, 10).
Increased myelin content at posterior thalamic radiation-related regions of
this radiation (RLIC) were detected in this study (11).
3) corpus callosum and tapetum participated in attention and auditory
information transfer (12).
Hence, increased myelination at visual and attention pathway provides evidence
of the dysfunction of visual processing and spatial awareness in ADHD.
Conclusion
Increased
myelin content was underscoring the important pathway of frontostriatal
tract, posterior thalamic radiation, and corpus callosum underlying
ADHD,
which reinforced the insights into myelin
quantification and its potential role in pathophysiological
mechanism and disease diagnosis. Besides,
SyMRI provides a more direct and sensitive method than DTI to estimation of
white matter microstructure alteration (e.g., myelin) on individual level.
Acknowledgements
This work was supported by the Natural Science Fund Youth Science Fund Project of China [grant number 82001439], the Natural Science Fund Project of Guangdong Province [grant numbers 2022A1515011910]. We would like to thank the participants and their families and the staff at the MRI at the First Affiliated Hospital of Sun Yat-sen University for all their help and support.References
1. Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA (2014): ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International journal of epidemiology. 43:434-442. 2. van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, et al. (2014): Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 53:790-799.e793. 3. Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in biomedicine. 15:435-455. 4. Hagiwara A, Warntjes M, Hori M, Andica C, Nakazawa M, Kumamaru KK, et al. (2017): SyMRI of the Brain: Rapid Quantification of Relaxation Rates and Proton Density, With Synthetic MRI, Automatic Brain Segmentation, and Myelin Measurement. Investigative radiology. 52:647-657. 5. Chen Y, Su S, Dai Y, Wen Z, Qian L, Zhang H, et al. (2021): Brain Volumetric Measurements in Children With Attention Deficit Hyperactivity Disorder: A Comparative Study Between Synthetic and Conventional Magnetic Resonance Imaging. Frontiers in neuroscience. 15:711528. 6. Yoo RE, Choi SH, Youn SW, Hwang M, Kim E, Oh BM, et al. (2022): Myelin Content in Mild Traumatic Brain Injury Patients with Post-Concussion Syndrome: Quantitative Assessment with a Multidynamic Multiecho Sequence. Korean journal of radiology. 23:226-236. 7. Chiang HL, Chen YJ, Lo YC, Tseng WY, Gau SS (2015): Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention-deficit/ hyperactivity disorder. Journal of psychiatry & neuroscience : JPN. 40:325-335. 8. Gau SS, Tseng WL, Tseng WY, Wu YH, Lo YC (2015): Association between microstructural integrity of frontostriatal tracts and school functioning: ADHD symptoms and executive function as mediators. Psychological medicine. 45:529-543. 9. Castellanos FX, Proal E (2012): Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends in cognitive sciences. 16:17-26. 10. Nazari MA, Berquin P, Missonnier P, Aarabi A, Debatisse D, De Broca A, et al. (2010): Visual sensory processing deficit in the occipital region in children with attention-deficit / hyperactivity disorder as revealed by event-related potentials during cued continuous performance test. Neurophysiologie clinique = Clinical neurophysiology. 40:137-149. 11. Sherbondy AJ, Dougherty RF, Napel S, Wandell BA (2008): Identifying the human optic radiation using diffusion imaging and fiber tractography. Journal of vision. 8:12.11-11. 12. Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract-based atlas of human white matter anatomy. Radiology. 230:77-87.Figures
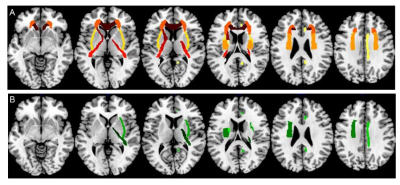
Figure 1 Significant ROIs of SyMRI-based myelin
parameters (A: MVF; B: MYV) between ADHD and TD
children Note. ROIs = region of interests; SyMRI = synthetic MRI; MVF =
myelin volume fraction; MYV = myelin volume; ADHD =
Attention-deficit/hyperactivity disorder; TD = typically developing.
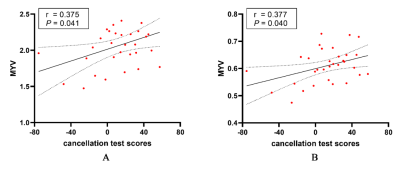
Figure 2 The
results of correlations analysis of the significant myelin parameters and
neuropsychologic tests in ADHD. Note. The regional MYV at left SCR (A) was
positively correlated with cancellation test scores (r = 0.375, P =
0.041), and the regional MYV in the right PLIC (B) was positively associated
with cancellation test scores (r = 0.377, P = 0.040). ADHD =
Attention-deficit/hyperactivity disorder; MYV = myelin volume; ACR = anterior
corona radiata; PLIC = posterior limb of internal capsule.
DOI: https://doi.org/10.58530/2023/2644