2639
Episodic menstrual migraine patients exhibit white matter microstructuralchanges compared to hormonal controls
1Institute for Systems and Robotics - Lisboa and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, Portugal, 2Universidad Autónoma de Madrid, Madrid, Spain, 3Learning Health, Hospital da Luz, Lisbon, Portugal, 4Imaging Department, Hospital da Luz, Lisbon, Portugal, 5Neurology Department, Hospital da Luz, Lisbon, Portugal, 6Center for Interdisciplinary Research in Health, Universidade Católica Portuguesa, Lisbon, Portugal
Synopsis
Keywords: White Matter, Diffusion/other diffusion imaging techniques, Migraine
Migraine is one of the most prevalent brain disorders worldwide. Although features extracted from diffusion MRI have been suggested to hold potential as disease biomarkers, research outputs remain inconsistent across studies. We investigated voxelwise microstructural alterations in episodic menstrual migraine patients (interictal phase) and appropriate hormonal controls (post-ovulation) by comparing diffusion-tensor and diffusion-kurtosis imaging metrics. Moreover, we extracted histogram measures (median, peak height, width, and value for each metric); and we evaluated their relationship with clinical factors (disease duration, attack frequency and pain intensity). Several metrics revealed significant differences between groups, indicating that they may be potential disease biomarkers.Introduction
Migraine is a brain disorder characterized by recurrent attacks of moderate to severe headache1. Although it is one of the most common diseases worldwide, affecting approximately 12% of the population and causing significant disability, there is still an unmet need for neuroimaging biomarkers2. Several studies have shown that diffusion MRI (dMRI) may provide sensitive biomarkers reflecting microstructural white matter (WM) changes3. However, results are somehow inconsistent, which might be due to the heterogeneous composition of the cohorts of migraine patients in each study, including multiple migraine subtypes without appropriate controls4. Here, we chose to focus on a very common subtype of episodic migraine, which is related with the menstrual cycle: menstrual migraine without aura, and used an appropriate hormonal control group. We analyzed WM microstructural changes in both groups by evaluating diffusion-tensor imaging (DTI) and diffusion-kurtosis tensor (DKI) parameters obtained from a multi-shell dMRI acquisition.Methods
Data acquisition:A group of 14 women with episodic menstrual migraine without aura (30±7yrs) were scanned during the interictal phase, and a control group of 15 healthy women (29±10yrs) were scanned in the corresponding phase of their menstrual cycle (post-ovulation). We collected 2D-EPI multi-shell dMRI data on a 3T Siemens Vida system with a 64-channel RF-receive head coil with the following parameters: TR/TE=6800/89ms, 66 slices, in-plane GRAPPA factor 2, SMS factor 3, 2mm isotropic resolution; with b=400,1000,2000s/mm2 along 32,32,60 gradient directions; and 8 b0s.
Data preprocessing & analysis:
Data were prepocessed following the DESIGNER pipeline5. We estimated DTI/DKI parameters using DESIGNER to obtain the following parameteric maps: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), mean kurtosis (MK), axial kurtosis (AK) and radial kurtosis (RK). Then, each parametric map was skeletonised using tract-based spatial statistics (TBSS)6. First, we employed voxelwise analysis to compare migraine patients against healthy controls (permutation testing, 5000 permutations, cluster-based correction; p<0.05)7. For the parameters exhibiting significant voxelwise group differences (MD, AD, MK and AK), we computed histograms (using R (r-project.org/); 1000 bins) across the skeletonized maps, and extracted the following metrics: median, peak height, width, and value. The computed histogram metrics were compared across groups with the Mann-Whitney test (applying Bonferroni corrections for multiple comparisons, p<0.05). In the patient group, we also investigated whether these histogram metrics were correlated (Spearman correlation) with clinical variables (disease duration; attack frequency and pain intensity) on JASP (jasp-stats.org/).
Results
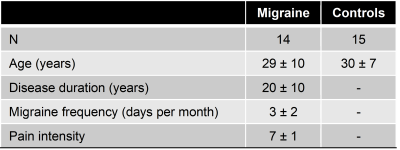
Theparticipants’ demographic information is summarized in Table 1. Overall, we
found that migraine patients had lower MD and AD values than controls, whereas no differences were
found in FA or RD. Interestingly, migraine patients also showed increased MK
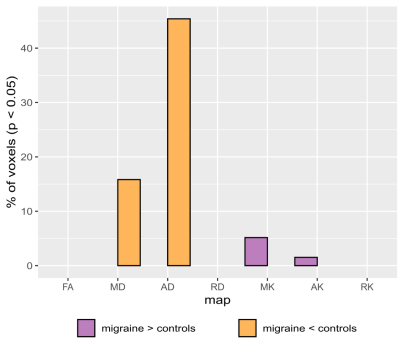
and AK, and no differences in RK. Figure 1 shows the percentage of voxels in
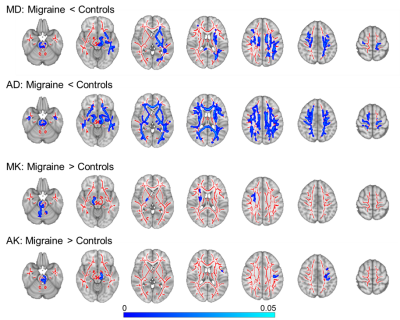
the mean FA skeleton with significant differences between groups (p<0.05), for each diffusion parameter. Figure 2 shows the spatial distribution of the
changes between groups for each
parameter that exhibit significant differences. Group differences were
found in the following brain regions: medial lemniscus, superior cerebellar
peduncle, corona radiata and superior longitudinal fasciculus. The distributions
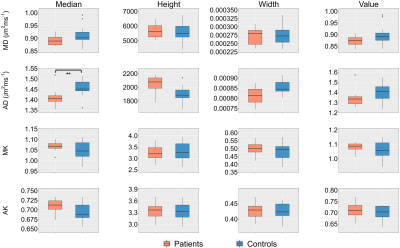
across patients and controls of all histogram-metrics for MD, AD, MK and AK are
presented in Figure 3. Significant group differences were found for AD median (p=0.016).
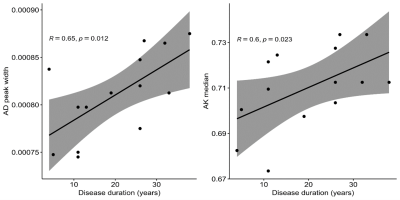
Regarding the correlation analysis represented in Figure 4, AD peak width
(r=0.65; p=0.012) and AK median (r=0.60; p=0.023) were positively associated
with disease duration. However, these results did not survive correction for multiple
comparisons.
Discussion/Conclusion
We found WM microstructural changes across multiple brain regions in patients with episodic menstrual migraine compared with hormonal controls. We found decreased MD and AD in patients, in agreement with the literature3. We also found increased AK and MK, which agrees with the only previous report of DKI parameters in migraine that we are aware of8. In that study, AK was increased in episodic migraine and RK was decreased in chronic migraine. Consistently, our results only showed differences in AK. Decreased AD was also found when considering the skeleton histogram median, while the other parameter differences were not reflected in the respective histogram metrics, probably because they were more localized (less diffuse across WM). Overall, our findings provide further support to the value of DTI/DKI parametric maps and derived histogram-metrics as potential biomarkers of migraine, showing that they exhibit clear changes in a very specific subtype of episodic migraine, related with the menstrual cycle. Future work will further investigate whether these changes in WM microstructure vary across the different phases of the migraine cycle.Acknowledgements
Weacknowledge the Portuguese Science Foundation through grants
SFRH/BD/139561/2018, PTDC/EMD-EMD/29675/2017, LISBOA-01-0145-FEDER-029675 and
UIDB/50009/2020.
References
1. P. J. Goadsby, et al., Physiol Rev, vol. 97, no. 2, pp. 553–622. 2017.
2. V. L. Feigin et al., Lancet Neurol, vol. 18, no. 5, pp. 459–480, 2019.
3. R. Rahimi et al., Brain Imaging and Behavior, 1-27, 2022.
4. Á. Planchuelo Gómez, “A multimodal analysis of Magnetic Resonance Imaging for the study of brain abnormalities in migraine: gray matter morphometry, white matter integrity and structural connectivity.”, PhD Dissertation, Universidad de Valladolid, Spain, 2021
5. B. Ades-Aron et al., Neuroimage, vol. 183, no. July, pp. 532–543, 2018.
6. S. M. Smith et al., Neuroimage, vol. 31, no. 4, pp. 1487–1505, 2006.
7. A. M. Winkler, et al., Neuroimage, vol. 92, pp. 381–397, 2014.
8. B. Ades-aron et al., in ISMRM 27th Annual Meeting, 2019.
Figures