2638
Automatic detection of cognitive impairment in patients with White matter hyperintensity based on deep learning and radiomics of MRI1Medical Imaging Department, Chongqing University Central Hospital, Chongqing, China, 2The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China, 3Shanghai United Imaging Intelligence, Co., Ltd., Shanghai, China
Synopsis
Keywords: White Matter, Machine Learning/Artificial Intelligence
White matter hyperintensity (WMH) is common in the aging brain, which is associated with cognitive decline and dementia. At present, there is still no objective method for early detection of cognitive impairment from these populations. In this study, deep learning and radiomics techniques were used to automatically segment and extract the characteristics of WMH and other regional brain tissues, and models were established to detect mild cognitive impairment.Introduction
White matter hyperintensities (WMH) (1) are the hyperintense patches on T2-weighted or fluid attenuated inversion recovery (FLAIR) images. It often appears in the aging brain and is considered to be the neuroimaging feature of cerebrovascular diseases, closely related to cognitive decline and dementia.Some studies have found thatthe WMH burden may be one of the early pathological changes related to the decline of cognitive ability in the elderly. High WMH load is an indicator of cognitive decline and dementia in the future. If individuals with cognitive impairment in WMH can be identified as early as possible, it is possible to prevent them from further deterioration, which will be of great significance for the prevention, treatment and prognosis of dementia.Therefore, an efficient and accurate detection method is urgently needed to identify and segment the internal structure of WMH and detect MCI individuals in WMH population.In the past (2), WMH was evaluated mainly by Fazekas score and WMH volume。Some studies have shown that fazekas classification is related to MCI. However, in clinical practice, some patients with WMH fazekas 2 can be combined with MCI, while some patients with WMH fazekas 3 can not be combined with MCI (3). On the other hand, the naked eye can only observe some simple parameters such as conventional diameter and morphology of WMH, and can not analyze the subtle changes in the internal structure of WMH. Previous studies have found that on the microscopic level, WMH is more likely to occur due to the low perfusion of white matter and the destruction of microstructure than conventional neuroimaging.The penumbra of white matter, which is visually normal in nature, has also been linked to cognitive impairment. Some studys (4,5)demonstrated the existence of penumbra by predicting the progress of wmh by radiomics. The 1cm penumbra area around WMH is the closest anatomical structure to WMH and can occur in any area around WMH. On the other hand, previous studies have found that atrophy of the cerebral cortex and deep brain nuclei is also associated with cognitive impairment in patients.
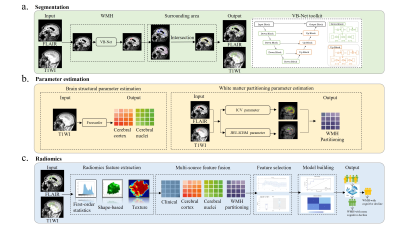
In this study, patients with mci(WMH-MCI) and non-MCI(WMH-NMCI) with WMH fazekas scores of 2 or 3 were included. WMH identification, segmentation, expansion and features extraction were automatically perfomed with a VB-net evolved from U-Net network. Radiomics was used to analyze the features. features of the cerebral cortex and nucleus were obtained using freesurfer tools. Four machine learning methods are used to build the training model and verify it in an independent external verification set.
Methods:
79 patients from center 1 were random divided into training set (62 patients) and testing set (17 patients). In addition, 29 patients from center 2 were included as an independent testing set. Freesurfer tool was used to obtain cerebral cortex and cerebral nuclei features. WMH identification, segmentation and features extraction were automatically perfomed with a VB-net evolved from U-Net network. White matter partition template were used to obtain the white matter volume, WMH volume and the proportion of WMH in the white matter of different partitions. After dimensionality reduction, four independent ML classifiers, including logistic regression, Gaussian process, random forest and quadratic discriminant analysis algorithm were trained on the training set, and the model parameters were adjusted on the testing set. Model performance were evaluated using area under the curve (AUC) index of receiver operating characteristics and compared with delong test.Results:
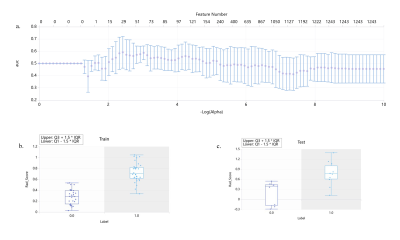
A total of 2264 radiomics features were automatically extracted from each WMH and the surrounding area ROI of each patient. After dimensionality reduction, 29 features were found associated with WMH-with cognitive decline. The diagnostic model of LR showed the best performance, the AUC on the training dataset, internal test dataset and external test dataset were 0.99, 0.843, 0.824, respectively. DeLong test shows that LR model has the highest accuracy among all models.Conclusion:
The LR model based on textural features of MRI images can detect cognitive impairment in WMH patients at an early stage.Key Points:
Radiomic features of WMH on MRI images have the potential to automatically detect cognitive impairment.Our artificial intelligence (AI) technology can accurately and automatically identify, segment and extract radiological features of WMH, establish machine learning models, and realize automatic detection of WMH-with cognitive decline patients.
The established LR model based on T1-weighted images showed the best performance and could be used as an auxiliary diagnostic tool for the evaluation of WMH-with cognitive decline patients.
Acknowledgements
References
1 Sassi C. White matter hyperintensities and neurodegenerative dementias. Aging (Albany NY). 2019 May 18;11(10):2912-2913. doi: 10.18632/aging.101967. PMID: 31102504; PMCID: PMC6555460.
2 Wardlaw JM, Smith EE, Biessels GJ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013 Aug;12(8):822-38. doi: 10.1016/S1474-4422(13)70124-8. PMID: 23867200; PMCID: PMC3714437.
3 Shu Z, Xu Y, Shao Y, Pang P, Gong X. Radiomics from magnetic resonance imaging may be used to predict the progression of white matter hyperintensities and identify associated risk factors. Eur Radiol. 2020 Jun;30(6):3046-3058. doi: 10.1007/s00330-020-06676-1. Epub 2020 Feb 21. PMID: 32086580.
4 Shao Y, Chen Z, Ming S, Ye Q, Shu Z, Gong C, Pang P, Gong X. Predicting the Development of Normal-Appearing White Matter With Radiomics in the Aging Brain: A Longitudinal Clinical Study. Front Aging Neurosci. 2018 Nov 28;10:393. doi: 10.3389/fnagi.2018.00393. PMID: 30546304; PMCID: PMC6279861.924990; PMCID: PMC8671609.
5 Shu ZY, Shao Y, Xu YY, Ye Q, Cui SJ, Mao DW, Pang PP, Gong XY. Radiomics nomogram based on MRI for predicting white matter hyperintensity progression in elderly adults. J Magn Reson Imaging. 2020 Feb;51(2):535-546. doi: 10.1002/jmri.26813. Epub 2019 Jun 11. PMID: 31187560.
Figures