2637
Alterations in white matter integrity of the pain modulatory system in patients with fibromyalgia
Yu-Ting Huang1, Ting-Chun Lin2, Yao-Wen Liang2, You-Yin Chen2,3, Jiunn-Horng Kang4,5,6, and Yu-Chun Lo3
1Department of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Taipei, Taiwan, 3Ph.D. Program in Medical Neuroscience, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan, 4College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan, 5Department of Physical Medicine and Rehabilitation, Taipei Medical University Hospital, Taipei, Taiwan, 6Professional Master Program in Artificial Intelligence in Medicine, College of Medicine, Taipei, Taiwan
1Department of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Taipei, Taiwan, 3Ph.D. Program in Medical Neuroscience, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan, 4College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan, 5Department of Physical Medicine and Rehabilitation, Taipei Medical University Hospital, Taipei, Taiwan, 6Professional Master Program in Artificial Intelligence in Medicine, College of Medicine, Taipei, Taiwan
Synopsis
Keywords: White Matter, Diffusion Tensor Imaging
The relationship between abnormal pain sensation and alterations in the central nervous system of fibromyalgia patients is unknown. In this study, diffusion tensor imaging was used to systematically investigate the alterations in white matter tracts of the pain modulatory pathways. The findings revealed the altered integrity of spinothalamic tract-thalamus, thalamus-insula tracts, tracts connected thalamus, midbrain and pons, were associated with the physical and psychological dysfunction in patients with fibromyalgia.Introduction
Fibromyalgia (FM) is the most frequent cause of persistent, widespread musculoskeletal pain, together with fatigue, sleep, memory, and mood problems1.Researches showed the abnormal pain sensation of FM was caused by altering the way the brain and spinal cord process pain, further giving rise to the related mental disorder of FM patients2. There are three distinct but interconnected pathways in the anatomical processing of chronic pain. The sensory aspects of pain, including location, characteristics, and intensity, are encoded via the lateral pathway. The affective-motivational components of pain are transmitted through the medial pathway. In addition to the two ascending pathway, the descending pain inhibitory pathway represents the ability to suppress pain and regulates context-dependent pain perception such as placebo analgesia3. Diffusion tensor imaging (DTI) is frequently used to analyze the white matter (WM) architecture in the brain non-invasively and provide clues of disease progress and clinical consequences4. The change of WM integrity in the brain regions related to pain processing with DTI metrics, such as corpus callosum, cingulum, and thalamus, which was associated with clinical pain intensity and pain perception in FM patients5-7. However, there is no systematic analysis on how WM microstructures are altered through the three specific pain pathways in FM patients. Thus, we hypothesized alterations of WM integrity in pain modulatory pathways was related to process of abnormal pain sensation and alterations in the central nervous system of FM patients.Methods
Thirty-eight FM patients (4 males / 34 females, mean age = 48.95±11.13 years) and forty-one healthy control participants (HC) (5 males / 36 females, mean age = 47.17±12.79 years) were included. Patients were confirmed by experienced rheumatologists and met the American College of Rheumatology criteria for FM8. Whole brain images were obtained using a 3 Tesla MRI (Siemens, Prisma, Munich, Germany). DTI was acquired using the spin-echo EPI method with a b value of 1000 s/mm2 for each of 64 diffusion encoding directions, and one b0 image set, (TR/TE=7000/65 ms, FOV=196×196mm2, Matrix:128×128×63, 3-mm slice thickness). The in-plane resolution was 1.53125 mm. To acquire the spin distribution function, the diffusion data were reconstructed by using q-space diffeomorphic reconstruction in the MNI space. The targeted tracts were reconstructed using DSI Studio (http://dsi-studio.labsolver.org). The WM integrity in each targeted tract was measured by fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) values. Regions of interests (ROIs) in both hemispheres shown in Figure 1 were selected according to the three pain pathways according to previous study3. FA, MD, AD, RD, and demographic were compared between patients with FM and HC group using independent two-sample t-test. P values (2-tailed) under 0.05 were regarded as significant. Data are presented as mean ± standard error of the mean. All calculations were performed with SPSS V.26 (Armonk, NY: IBM Corp).Results
No significant difference of demography between the FM patients and the HC group. Significant higher AD and RD were found in the left spinothalamic tracts (STT) to thalamus in the lateral and medial pathway in the FM group compared with the HC group (Figure 2). In the medial pathway, FA in right thalamus-insula, MD and AD in left thalamus-insula significantly decreased in the FM group (Figure 3). As compared to the HC group, significant higher MD, AD, and RD were revealed in the left thalamus-midbrain-pons of the inhibitory pathway (Figure 4).Discussion
Significantly higher RD were found in the left STT-thalamus of the FM group. Elevated RD is associated with demyelination9. Demyelinated axons may discharge action potentials spontaneously10 and were associated with the pain of multiple sclerosis11. Studies show multiple sclerosis lesions that grow in the spinothalamic tract of the cervical and thoracic spinal cord cause central extremity pain, therefore suggesting the spinothalamic tract is where extremities neuropathic pain originates12.Significantly lower MD and AD were found in the left thalamus-insula of the FM group, and significantly lower FA was found in the right thalamus-insula. Neuronal damage and loss, which cytotoxic edema is associated with the decrease of MD and axonal loss correlates to decreased AD, possibly indicated hampered connections with the insular cortex13, 14. Insula is considered a limbic-related cortex, and anterior insula directly corresponds with all emotional feelings and subjective physical sensations15. The axonal pathology along the WM tracts from thalamus to insula is correlated with abnormal affective regulation processes such as bipolar disorder and obsessive-compulsive disorder16, 17. Similar psychological alterations such as anxiety and depression can be seen on FM patients18, 19.
Significantly higher MD, AD and RD were found in the left thalamus-midbrain-pons of the FM group. In the inhibitory pathway, the periaqueductal gray (PAG) of the midbrain is a crucial structure in the propagation and modulation of pain20. PAG has been shown to have an antinociceptive role with inhibitory connections to the trigeminal sensory nucleus and the dorsal horn of the spinal cord21. Chronic pain such as FM is associated with the result of deficiency in activation of the pain inhibitory pathway3.
Conclusion
In this study, we successfully investigated the WM microstructure in three pain modulatory pathways in FM patients has shown difference compared with HC group, which might be a potential neural substrate in fibromyalgia.Acknowledgements
This work is financially supported by Ministry of Science and Technology of Taiwan under Contract numbers of MOST 110-2314-B-038-052, 108-2314-B-038-101-MY2, and 110-2314-B-038-074. We also are grateful for support from Imaging Center for Integrated Body, Mind and Culture Research, National Taiwan University.References
1. Clauw DJ. Fibromyalgia: a clinical review. JAMA. Apr 16 2014;311(15):1547-1555.2. Arnold LM, Bennett RM, Crofford LJ, et al. AAPT Diagnostic Criteria for Fibromyalgia. J Pain. Jun 2019;20(6):611-628.
3. De Ridder D, Vanneste S, Smith M, Adhia D. Pain and the Triple Network Model. Frontiers in Neurology. 2022:757241.
4. Poretti A, Meoded A, Rossi A, Raybaud C, Huisman TA. Diffusion tensor imaging and fiber tractography in brain malformations. Pediatr Radiol. Jan 2013;43(1):28-54.
5. Aster H-C, Evdokimov D, Braun A, et al. CNS imaging characteristics in fibromyalgia patients with and without peripheral nerve involvement. Scientific Reports. 2022/04/25 2022;12(1):6707.
6. Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin. 2013;3:249-260.
7. Kim DJ, Lim M, Kim JS, Son KM, Kim HA, Chung CK. Altered white matter integrity in the corpus callosum in fibromyalgia patients identified by tract-based spatial statistical analysis. Arthritis Rheumatol. Nov 2014;66(11):3190-3199.
8. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. Dec 2016;46(3):319-329.
9. Ranzenberger LR, Snyder T. Diffusion Tensor Imaging. StatPearls. 2022.
10. Finnerup NB, Kuner R, Jensen TS. Neuropathic Pain: From Mechanisms to Treatment. Physiol Rev. Jan 1 2021;101(1):259-301.
11. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. Jan 2013;13(1):320.
12. Scherder RJ, Kant N, Wolf ET, Pijnenburg BCM, Scherder EJA. Sensory Function and Chronic Pain in Multiple Sclerosis. Pain Res Manag. 2018;2018:1–9.
13. Zhang X, Sun P, Wang J, Wang Q, Song SK. Diffusion tensor imaging detects retinal ganglion cell axon damage in the mouse model of optic nerve crush. Invest Ophthalmol Vis Sci. Sep 1 2011;52(9):7001-7006.
14. Palesi F, Castellazzi G, Casiraghi L, et al. Exploring Patterns of Alteration in Alzheimer's Disease Brain Networks: A Combined Structural and Functional Connectomics Analysis. Front Neurosci. 2016;10:380.
15. Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. Apr 2011;1225:72-82.
16. Canales-Rodriguez EJ, Pomarol-Clotet E, Radua J, et al. Structural abnormalities in bipolar euthymia: a multicontrast molecular diffusion imaging study. Biol Psychiatry. Aug 1 2014;76(3):239-248.
17. Huang BL, Wang JR, Yang XH, Ren YM, Guo HR. A study on diffusion tensor imaging in patients with untreated first-episode obsessive-compulsive disorder. Quant Imaging Med Surg. Feb 2022;12(2):1467-1474.
18. Izquierdo-Alventosa R, Ingles M, Cortes-Amador S, et al. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int J Environ Res Public Health. May 21 2020;17(10):3634.
19. Hauser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. Mar 2018;20(1):53-62.
20. Mokhtar M, Singh P. Neuroanatomy, Periaqueductal Gray. StatPearls. 2022.
21. Fiore A, Tsantes E, Curti E, Bazzurri V, Granella F. Secondary cluster headache due to a contralateral demyelinating periaqueductal gray matter lesion. Headache. Jul 2021;61(7):1136-1139.
Figures
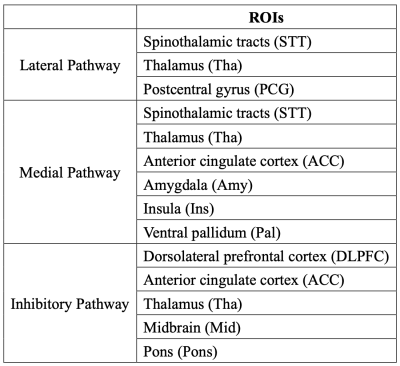
Selection of ROIs. The exact listing of all regions of interest used in the three pain pathways.
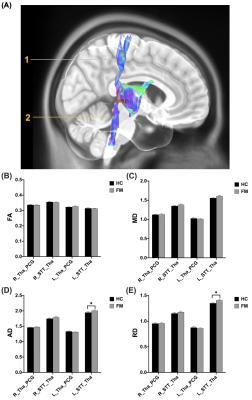
(A) The visualization of the WM tracts in the lateral pathway (1: PCG-Tha, 2: Tha-STT). (B) FA and (C) MD values showed no significant differences between HC and FM groups. In the left STT-Tha WM tracts of the lateral pathway, (D) AD values showed significant differences (HC: 1.9471±0.0207, FM: 2.0091±0.0216, p = 0.042), and (E) RD also reported significant differences (HC: 1.3503±0.0192, FM: 1.4078±0.0199, p = 0.041) between HC and FM groups.
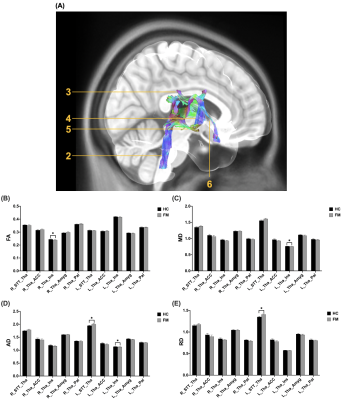
(A) The visualization of the WM tracts in the medial pathway (2: Tha-STT, 3: Tha-ACC, 4: Tha-Pal, 5: Tha-Amy, 6: Tha-Ins). Significant difference can be obtained in (B) FA of the right Tha-Ins WM tracts (HC: 0.24480.0022, FM: 0.23910.0017, *p=0.047), (C) MD of the left Tha-Ins WM tracts (HC: 0.76440.0037, FM: 0.75530.0024, p=0.045), AD of the left STT-Tha (HC: 1.94710.0207, FM: 2.00910.0216, p = 0.042) and the left Tha-Ins WM tracts (HC: 1.13940.0047, FM: 1.12760.0036, p=0.050), and RD in the left STT-Tha WM tracts (HC: 1.35030.0192, FM: 1.40780.0199, p=0.041).
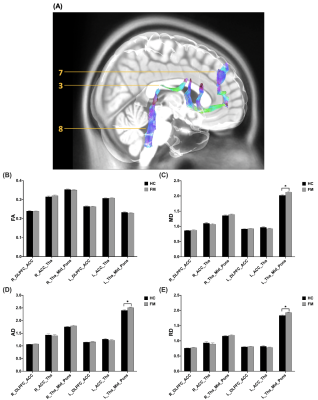
(A) The visualization of the WM tracts in the inhibitory pathway (3: Tha-ACC, 7: DLPFC-ACC, 8: Tha-Mid-Pons). (B) FA values showed no significant differences in the inhibitory pathway. In the left Tha-Mid-Pons WM tracts of the inhibitory pathway, significant differences can be obtained in (C) MD values (HC: 2.0217±0.0226, FM: 2.1280±0.0215, p=0.001), (D) AD values (HC: 2.4036±0.0254, FM: 2.5088±0.0230, p=0.003), and (E) RD values (HC: 1.8370±0.0203, FM: 1.9288±0.0195, p=0.002).
DOI: https://doi.org/10.58530/2023/2637