2626
The effects of magnetization transfer on fast and slow diffusion compartments in myelinated white matter1Department of Radiology, NYU Grossman School of Medicine, New York, NY, United States, 2Vilcek Institute of Graduate Biomedical Sciences, NYU Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: White Matter, Contrast Mechanisms
Myelin, although not directly visible in conventional diffusion MRI (dMRI) due to its short T2, still affects the dMRI signal indirectly through exchange processes with nearby water compartments. In this study, we investigated how a magnetization transfer (MT) preparation affects dMRI signals, in particular, the fast and slow diffusion compartments in myelinated white matter of ex vivo mouse brain. Our result demonstrated that the MT preparation altered the volume fractions, but not the diffusivities, of the fast and slow compartments. Examining the change in volume fractions with MT preparation can provide insights into exchange processes involving myelin and water compartments.Introduction
The diffusion MRI (dMRI) signals in white matter (WM) exhibits non-mono-exponential decay with increasing diffusion weighting1, and is often separated into signals from a fast and a slow diffusion compartment, commonly ascribed to the extra-axonal and intra-axonal spaces in the direction transverse to the WM fibers. In myelinated white matter (WM), the myelin sheath physically separates these two spaces, with continuous exchanges of water and spin saturation at the interfaces between myelin and other water compartments2,3.Magnetization Transfer (MT) uses off-resonance pulses to saturate semisolids in tissue (e.g. myelin), and the saturation can be transferred to nearby water via several exchange processes4-6. By adding MT preparation, we aim to examine how exchange with myelin affect dMRI signals from the fast and slow compartments. We hypothesized that the MT preparation will affect signals from the two compartments differently, and the difference may shed new light on the interactions between myelin and nearby water compartments in WM.
Methods
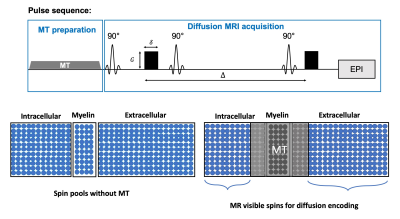
All experimental procedures were approved by the Institutional Animal Care and Use Committee. Data were acquired from ex vivo C57BL/6 mouse brains (P56, n=4) at room temperature. MT prepared diffusion MRI (Fig. 1) data was acquired using the following parameters: single-shot EPI, resolution = 0.1×0.1×1.5 mm3, 1.5 mm slice thickness, TR/TE = 5000/35ms, 4/35ms with 15 b values range up to 7.0 μm2/ms and diffusion direction perpendicular to axons in the corpus callosum. MT pulses train (gaussian pulses, bandwidth=1.5kHz, offset frequency=3kHz, peak amplitude=8μT) was placed before the excitation pulse. The MT-dMRI signals from the corpus callosum were fitted to a modified two-compartment model $$$ \frac{S_{1}(MT)}{S_{0}(MT)}=f_{s}(MT)\cdot e^{-b\cdot D_s(MT)}+(\frac{S_{0}(MT)}{S_{0}(0)}-f_{f}(MT))\cdot e^{-b\cdot D_f(MT)}$$$ , where the non-diffusion-weighted and diffusion weighted signals ($$$S_{0}$$$ and $$$S_{1}$$$), diffusivities ($$$D_{s}$$$ and $$$D_{f}$$$) and volume fractions ($$$f_{s}$$$ and $$$f_{f}$$$) for the fast and slow compartments are functions of MT-preparation.Results
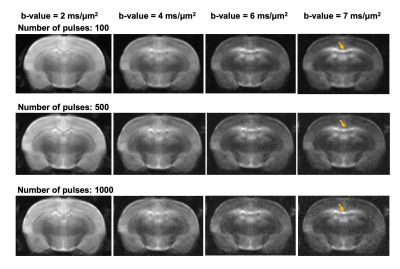
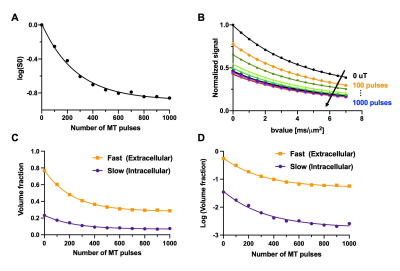
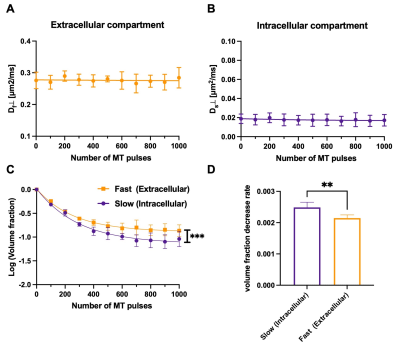
Representative MT-dMRI images showed signals in the corpus callosum stayed above noise floor even with strong diffusion and MT weighting (Fig. 2). $$$S_{0}(MT)$$$ from the corpus callosum decayed non-mono exponentially with increasing number of MT pulses, and $$$S_{1}(MT)$$$ decayed non-mono exponentially with increasing b-value (Fig. 3A-B). Fig. 3C shows the estimated $$$f_{s}(MT)$$$ and $$$f_{f}(MT)$$$ from the same subject.Although no apparent change in $$$D_{s}(MT)$$$ and $$$D_{f}(MT)$$$ was found with increasing number of MT pulses (Fig. 4A-B), the normalized $$$f_{s}(MT)$$$ (with respect to $$$f_{s}(0)$$$) decreased faster with increasing number of MT pulses than normalized $$$f_{f}(MT)$$$ (Fig. 4C, p < 0.001, paired ANOVA). The rate of decrease for $$$f_{s}(MT)$$$ (measured by the slope for the first 400 MT pulses) was about 25% higher than $$$f_{f}(MT)$$$ (Fig. 4D).
Discussion
We observe that MT-preparation does not affect $$$D_{s}$$$- and $$$D_{f}$$$, corresponding to the intra-, and extra-axonal compartmental diffusivities, but alters the corresponding compartmental fractions, with faster reduction in $$$f_{s}$$$ with increasing number of MT pulses. This observation suggests that (1) the slow diffusion or intra-axonal compartment is more tightly coupled with myelin than the fast diffusion or extra-axonal compartment (e.g faster exchange); (2) the MT pulses caused more direct signal attenuation in the slow diffusion compartment than the fast diffusion compartment. In this study, we assume that the MT-preparation will only suppress spins exchanging with myelin, but MT saturation is more complex due to the nonlinear characteristics of MT pulses5,6, presence of multiple exchange processes between myelin and nearby water compartments4, and direct suppression of free water pool by off-resonant pulses.Conclusion
Combining MT with dMRI allows us to examine MT-related signal attenuations in the fast and slow diffusion compartment separately.Acknowledgements
The project was supported by the National Institute of Health (NIH) R01 HD 074593, R01 NS 102904 and R01 NS088040, and was performed using shared resource supported byNIH 1S10OD018337-01, 5P30CA016087, and P41 EB017183.References
1. Stanisz, G. J., Szafer, A., Wright, G. A. & Henkelman, R. M. An analytical model of restricted diffusion in bovine optic nerve. Magnet Reson Med 37, 103-111 (1997). https://doi.org:DOI 10.1002/mrm.1910370115
2. Brusini, L., Menegaz, G. & Nilsson, M. Monte Carlo Simulations of Water Exchange Through Myelin Wraps: Implications for Diffusion MRI. IEEE Trans Med Imaging 38, 1438-1445 (2019). https://doi.org:10.1109/TMI.2019.2894398
3. van Gelderen, P. & Duyn, J. H. White matter intercompartmental water exchange rates determined from detailed modeling of the myelin sheath. Magnet Reson Med 81, 628-638 (2019). https://doi.org:10.1002/mrm.27398
4. van Zijl, P. C. M., Lam, W. W., Xu, J., Knutsson, L. & Stanisz, G. J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 168, 222-241 (2018). https://doi.org:10.1016/j.neuroimage.2017.04.045
5. Henkelman, R. M. et al. Quantitative interpretation of magnetization transfer. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 29, 759-766 (1993). https://doi.org:10.1002/mrm.1910290607
6. Sled, J. G. & Pike, G. B. Quantitative interpretation of magnetization transfer in spoiled gradient echo MRI sequences. J Magn Reson 145, 24-36 (2000). https://doi.org:10.1006/jmre.2000.2059
Figures