2625
MRI characterization of a mouse model of Staphylococcus aureus infection
Hannah Goldman1, Katherine Le2, Orlando Aristizabal1, Matias Aristizabal1, Mia Weissman1, Victor Torres2, and Youssef Zaim Wadghiri1
1Radiology, NYU Langone Health, New York, NY, United States, 2Microbiology, NYU Grossman School of Medicine, New York, NY, United States
1Radiology, NYU Langone Health, New York, NY, United States, 2Microbiology, NYU Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: Infectious disease, Preclinical
Staphylococcus aureus is of particular concern due to the deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains. There is not currently a vaccine as translation from preclinical models to humans has been unsuccessful. Acquiring reproducible and comparable spinal images that offer non-invasive visualization of the extent and impact of the infection are important for successful intervention or vaccine development. 3D-printed, MRI-compatible cradles enable reproducible positioning and analysis for both in vivo and ex vivo MR spinal imaging in mouse models while allowing for nondestructive analysis of the potential additional benefits to visualization associated with ex vivo MEMRI.Introduction
Staphylococcus aureus (S. aureus) is an opportunistic pathogen that is of particular concern due to the deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains such as methicillin-resistant S. aureus (MRSA)1. Despite relevant research in the area, vaccine candidates against S. aureus have not been successfully translated from preclinical models to humans. Imaging the extent and impact of infection can lead to better understanding and allow for visualization of potential successful intervention or vaccine development. There is current difficulty in acquiring reproducible and comparable spinal images due to the natural curvature of the spine and lack of non-invasive and/or non-paramagnetic/MRI-compatible solutions for reproducible positioning of the spine that would enable characterization using MR imaging. Use of 3D-printed cradles can provide low-cost, highly customizable prototyping with a quick and iterative design2. This work describes a solution devised to document this murine model using a workflow spanning from in vivo MR imaging to subsequent ex vivo acquisition with the latter enabling multi-contrast analysis with enhanced spatial resolution during unattended overnight scans. Additionally, the use of ex vivo manganese-enhanced MRI (MEMRI) was conducted to enhance the neuroarchitectural details and facilitate image analysis of complex morphological changes post-mortem.Methods
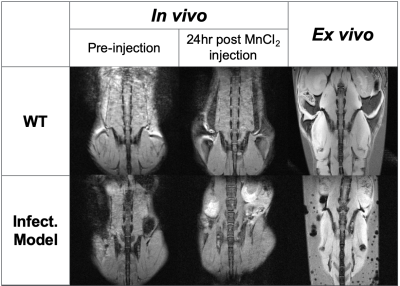
Both an infectious model and wildtype C57BL/6 (B6) mice were infected with a strain of the MRSA USA300 clone (AH-LAC) via lateral tail vein injection. At two days post-infection, bacteria were detected in the distal organs and tissues, including the spine, using fluorescence imaging and in vivo MRI. Following in vivo MRI, mice were transcardially perfused and placed in degassed 1x phosphate buffered saline in preparation for ex vivo imaging. For MEMRI, mice were injected intraperitoneally with manganese chloride tetrahydrate in isotonic saline 24 hours prior to imaging3. Following in vivo MEMRI imaging, mice were transcardially perfused with a paraformaldehyde/glutaraldehyde solution and prepared for re-imaging4. Both in vivo and ex vivo imaging was performed using the 3D printed cradle. Imaging was performed on a Bruker Biospec 7030 7-Tesla micro-MRI system interfaced to an Avance 3-HD console using a commercial 40-mm circularly-polarized volume birdcage coil from the same manufacturer. T1-weighted 3D multi-gradient echo (MGE) and fast-spin echo (FSE) were acquired both in vivo and ex vivo in order to compare spinal anatomy visualization.Results
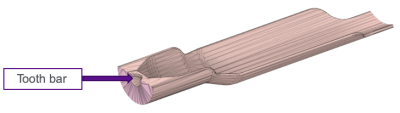
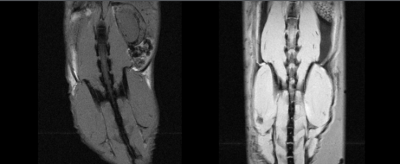
The 3D-printed cradle prototype (Figure 1) was able to accommodate mice of varying body mass and composition without affecting functionality. Securing of the animal to the cradle was sufficient to enable effective alignment of the spine (Figure 2) and allow for increased reproducibility and consistency between subjects without increasing the amount of set-up time required (Figure 3). The use of a paraformaldehyde/glutaraldehyde mixture as a fixative following manganese injection allowed for ex vivo T1-weighted signal enhancement comparable to that found in vivo4 (Figure 3). The use of ex vivo imaging for this study enabled acquisition of a dataset free of ghosting artifacts typically associated with this abdominal region due to breathing motion while lifting the limiting constraint of the in vivo imaging time window4. Consequently, ex vivo imaging performed during unattended overnight acquisition enabled greater sensitivity through signal accumulation resulting in enhanced anatomical details best suited for visualizing the complex morphology of the spine and allowing for analysis of the impact of infection in the S. aureus murine model.Discussion
The use of 3D printing technology allows for the development of highly specialized cradles through iterative design that can accommodate the necessary elements of any MRI study including coil configurations, physiological monitoring equipment, and preferred animal positioning. The reproducible images achieved with this new design helps decrease set-up time and improve throughput in a cost-effective manner. Furthermore, the use of the same cradle under both in vivo and ex vivo conditions enabled reliable co-registration between both datasets. Importantly, the proposed setup will help us characterize murine models of S. aureus within a spinal region where physiological motion and small cross-sectional dimensions can be challenging to delineate. The nondestructive nature of MRI enables multi-parametric ex vivo examination while maintaining tissue integrity. Our experimental setup and workflow will prove instrumental in characterizing mouse models, such as that of S. aureus, where large cohorts are needed due to variation in immune response and outcome. Furthermore, MEMRI proved valuable both in vivo and ex vivo for visualizing spinal anatomical features that can be challenging to delineate without contrast enhancement due to their anatomical size but further exploration is needed to determine the time window of greatest contrast increase in the spine post-manganese injection. Future directions on the refinement of cradle design are focused on the integration of sensors for physiological monitoring as well as the concomitant use of multiple receive-only phased array coils in order to perform dynamic contrast enhanced MRI using gadolinium in order to examine pharmacokinetics of the infected region.Acknowledgements
This work was supported, in part, by NIH grant R01 AI105129 and all MRI imaging was performed at the NYU Langone Health Preclinical Imaging Core, a shared resource partially supported by the NIH/SIG 1S10OD018337-01, the Laura and Isaac Perlmutter Cancer Center Support Grant NIH/NCI 5P30CA016087 and the NIBIB Biomedical Technology Resource Center Grant NIH P41 EB017183.
References
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-661. doi:10.1128/CMR.00134-14
- Donohoe, D.L., Dennert, K., Kumar, R. et al. Design and 3D-printing of MRI-compatible cradle for imaging mouse tumors. 3D Print Med 7, 33 (2021). doi: 10.1186/s41205-021-00124-6
- Silva AC, Bock NA. Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. Schizophr Bull. 2008 Jul;34(4):595-604. doi: 10.1093/schbul/sbn056.
- Liu Y, Sajja BR, Gendelman HE, Boska MD. Mouse brain fixation to preserve In manganese enhancement for ex vivo manganese-enhanced MRI. J MagnReson Imaging. 2013;38(2):482-487. doi:10.1002/jmri.24005
DOI: https://doi.org/10.58530/2023/2625