2624
Preserved white matter integrity despite changes in brain structure and metabolism in mouse with lethal irradiation and bone marrow transplant1Radiology/Gruss MRRC, Albert Einstein College of Medicine, Bronx, NY, United States, 2Cell Biology, Albert Einstein College of Medicine, Bronx, NY, United States
Synopsis
Keywords: White Matter, Transplantation, white matter, spectroscopy, irradiation, animal
Adult cells from transplanted bone marrow can generate new neurons in central nervous system (CNS) in rodent and human. Here we report for the first time in live mouse that white matter integrity in lethally irradiated mouse transplanted with adult mouse bone marrow (BMTX) is preserved, reflected in increased fractional anisotropy (FA) of corpus callosum post BMTX. Stable concentration of N-acetyl-aspartate (NAA) and increased FA after BMTX suggests that donor cells along with irradiation may stimulate new neuronal proliferation. Nevertheless, decreased myo-inositol concentration may reflect its cell volume regulation function as seen in morphometric adjustments in BMTX brains.Introduction
Bone marrow transplantation (BMTX) is a common treatment for malignant disorders and some non-malignant hematological conditions1,2. Total body irradiation (TBI) is a powerful tool to eradicate cells and to suppress the immune system to enable bone marrow engraftment3,4. Growth failure and undesirable neurological sequelae after BMTX and TBI have been reported in children5,6. However, it is still unknown if TBI has any adverse effects on brain white matter integrity and metabolism, especially in adult brains. Thus, we studied mouse brain via in vivo MRI/MRS on normal adult mice which were lethally irradiated and subsequently intravascularly injected with bone marrow from an adult donor mouse. The mice were studied prior to the BMTX and were followed up for 20 weeks post BMTX. Brain volumes, brain microstructure (DTI measures) and brain metabolites were studied. Because it has been reported in rodent and human that transplanted bone marrow cells can differentiate into brain cells and the bone marrow can migrate into the brains of adult mice rapidly giving rise to neurons there7,8, we hypothesized that the generation of neurons in the adult brain post BMTX may preserve white matter integrity in mouse brain.Methods
Eight C57BL/6J mice (4F/4M) were lethally irradiated with 1200 cGy in 2 split doses9, and subsequently injected with nucleated cells from the bone marrow of C57BL/6J donor mouse. We measured volumes (FSE), cerebral blood flow (CBF), brain microstructural integrity from Diffusion Tensor Imaging (DTI) fractional anisotropy (FA) and tissue inflammation (mean diffusivity, MD), using MRI/MRS at 9.4 Tesla (Agilent, Santa Clara, CA). Dorr mouse brain atlas10 was registered to T2-wt FSE images using ANTs11. DTI maps were generated using FSL diffusion toolbox. Volumes of segmented regions and average diffusion metrics over the segmented regions were then calculated. Metabolite concentrations in the thalamus were measured using MRS12. Mice were imaged prior to BMTX (baseline), and then imaged every 2 weeks till 20 weeks post BMTX. Another group of control C57BL/6J mice (n=5) were imaged at the same age as the BMTX mice at 20 weeks post BMTX, i.e., around 8 month old.Results
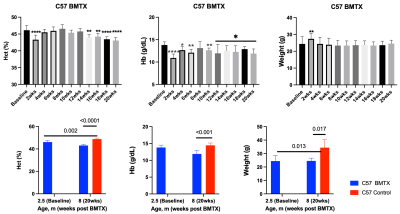
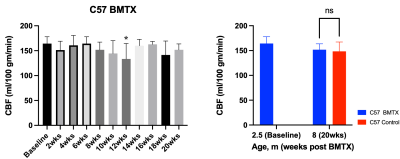
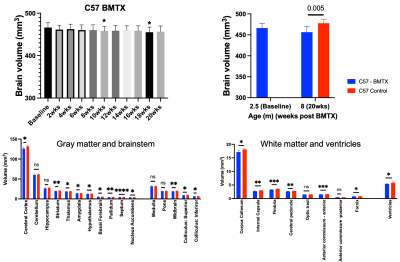
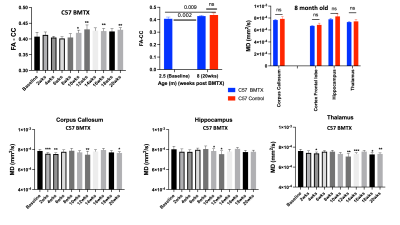
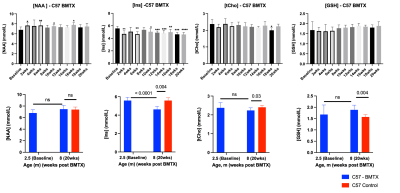
Both hematocrit and hemoglobin concentrations dropped post BMTX (Figure 1). BMTX growth post BMTX was reduced, and they were much lighter than C57 control mice at 8 months of age (Figure 1), probably due to growth hormone deficiency caused by TBI6,13. Despite lower Hct and Hb concentrations, CBF did not change significantly post BMTX. At age 8 months, CBF in BMTX mice was not different from that of control (Figure 2). Similar to smaller body weight in BMTX mouse, their brains were also smaller. Many grey and white matter brain regions as well as the ventricles were smaller in BMTX brains when compared to controls. However, volumes of cerebellum, hippocampus, medulla and pons in BMTX mouse brain were not different from controls’ (Figure 3). Interestingly, FA of corpus callosum (FA-CC) in BMTX mice was stable up to 8 weeks post-transplant, then began to increase starting 10 weeks post BMTX. At 8 month old, FA-CC in BMTX mouse brain is significantly higher than at their baseline (2.5 months of age) level, and was not different from that in control mouse brain (Figure 4) at 8 months of age. Mean diffusivity (MD) in BMTX mouse is not different from control mice at 8 months age (Figure 4). Concentration of myo-inositol (Ins) in thalamus decreased in BTMX mice from baseline to 20 weeks post BTMX, while concentrations of N-acetyl-aspartate (NAA), total choline (tCho) and glutathione (GSH) remained unchanged. However, [tCho] and [Ins] were both decreased at 20 weeks post BMTX in comparison to control mice at 8 months of age. Interestingly, [GSH] of BMTX mice 20 weeks post BMTX was higher than that of control mice at 8 month old (Figure 5).Discussion
Adult cells from bone marrow can generate new neurons in CNS7,8 in rodent and human. Our data here is the first to report in live mouse that BMTX in lethally irradiated normal mice has no detrimental effects on neurons. NMR-identified NAA is often considered specific for neurons, but despite the increasing FA post BTMX, NAA was not different from control mouse 20 weeks post BMTX (Figure 5). Importantly, neurometabolite reductions compared to controls at 20 weeks post BMTX did not appear to be related to brain perfusion, but may be related to the reduction in brain volume and might reflect a reduced cellular volume, also reflected in the decreased MD in thalamus where metabolite concentrations were estimated. The stability of [NAA] after BMTX may be attributed to increases in fatty acid synthesis and myelin synthesis14, as FA in white matter continues to increase post BMTX (Figure 4). Decreased concentrations of tCho and Ins with BMTX may reflect altered glia density, membrane phospholipid turnover and regulation of cell volume15-17, which are reflected in smaller BMTX brains.Conclusion
Stable NAA concentration and increasing FA post BMTX suggest that neurogenesis continues in adult BMTX mouse brain. Transplanted bone marrow cells may contribute to neuronal proliferation in adult mouse brain. Changes in cellular volumes and glial osmoregulatory functioning may result in decreased myo-inositol concentrations.Acknowledgements
No acknowledgement found.References
1. Sullivan KM, Parkman R, Walters MC. Bone Marrow Transplantation for Non-Malignant Disease. Hematology Am Soc Hematol Educ Program. 2000:319-338.
2. Thomas ED. Marrow transplantation for malignant disease. Am J Med Sci. 1987;294(2):75-79.
3. Leiper AD. Late effects of total body irradiation. Arch Dis Child. 1995;72(5):382-385.
4. Thomas ED. Total body irradiation regimens for marrow grafting. Int J Radiat Oncol Biol Phys. 1990;19(5):1285-1288.
5. Zajac-Spychala O, Pawlak MA, Karmelita-Katulska K, et al. Long-term brain status and cognitive impairment in children treated for high-risk acute lymphoblastic leukemia with and without allogeneic hematopoietic stem cell transplantation: A single-center study. Pediatr Blood Cancer. 2020;67(6):e28224.
6. Thomas BC, Stanhope R, Plowman PN, Leiper AD. Growth following single fraction and fractionated total body irradiation for bone marrow transplantation. Eur J Pediatr. 1993;152(11):888-892.
7. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775-1779.
8. Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003;100(3):1364-1369.
9. Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047-3051.
10. Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage. 2008;42(1):60-69.
11. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
12. Cui MH, Billett HH, Suzuka SM, et al. Corrected cerebral blood flow and reduced cerebral inflammation in berk sickle mice with higher fetal hemoglobin. Transl Res. 2022;244:75-87.
13. Mulcahy Levy JM, Tello T, Giller R, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer. 2013;60(4):700-704.
14. Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78(4):736-745.
15. Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265(2):54-84.
16. Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82(4):736-754.
17. Elberling TV, Danielsen ER, Rasmussen AK, Feldt-Rasmussen U, Waldemar G, Thomsen C. Reduced myo-inositol and total choline measured with cerebral MRS in acute thyrotoxic Graves' disease. Neurology. 2003;60(1):142-145.
Figures