2622
Dynamic dextran-enhanced CEST MRI reveals the size effect of BBB disruption associated with neuroinflammation1Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 2Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Kirby Research Center, Kennedy Krieger Institute, Baltimore, MD, United States, 4Institute for Cell Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Keywords: Contrast Agent, Molecular Imaging, Blood-Brain Barrier; Multiple Sclerosis
Prevailing imaging methods primarily focus on detecting blood-brain barrier (BBB) dysfunction utilising tracers of small molecular weight. Robust imaging methods suitable for assessing BBB permeability in the macro-molecular size range are still lacking. Our study aimed to develop and optimize a dextran-based MRI approach for detecting size-dependent BBB permeability. Demonstrated in an EAE MS mouse model, we established a dextran-based CEST MRI protocol for measuring the intracerebral distribution of non-labeled dextrans, validated the results using immunohistochemistry, and compared the CEST MRI results with conventional Gd-enhanced MRI.
INTRODUCTION
Mounting evidence shows that the BBB is compromised in multiple neurodegenerative disorders, including multiple sclerosis (MS)1,2 and Alzheimer's disease (AD)3,4. Currently, two methods are available in the clinic for assessing BBB integrity: 1) biofluid tests that measure the concentrations of specific biomarkers in the cerebrospinal fluid (CSF) and/or blood, and 2) MRI of imaging agents that cross the BBB and penetrate into the brain. The latter approach is more advantageous because it is non-invasive and can reveal the temporal and spatial pattern of BBB disruption. One of the potential pitfalls of the available method, however, is that BBB leakage is measured only in the lower molecular size range with the use of small molecular tracers, i.e., Gd-based agents (<1 kDa)5,6 and water (18 Da)7,8. The goal of this study is to develop non-labeled dextrans as a safe macromolecular CEST MRI agents for assessing BBB permeability in the larger molecular size range and use it as an effective tool to non-invasively study the size characteristics of BBB disruption associated with different neurodegenerative diseases.METHODS
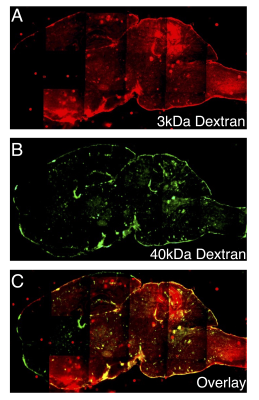
All animal experiments are approved by our Animal Care and Use Committee. EAE MS mouse model 9: Female C57Bl/6 mice, 6-10 w, were injected s.c. with myelin peptide (MOG35-55, 200 μL, 0.5 mg/mL) emulsified in incomplete Freund's adjuvant supplemented with M. tuberculosis H37Ra (5 mg/mL) and i.p. with 300 ng of pertussis toxin on days 0 and 2. Mice were observed daily for signs of paralysis using a 0-5 rating system with 0=healthy.Fluorescent imaging: EAE mice (n=3) were i.v. injected with a flexible combination of Dex40-TRITC (MW= 40 kDa) and Dex3-FITC (MW= 3 kDa) at 80 mg/kg. Mice were sacrificed 30 min after injection, and brains were collected and sectioned into 20 μm slices for fluorescence microscopy.
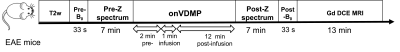
In vivo MRI: Three EAE mice were randomly selected to acquire MRI using a Biospec 11.7 T horizontal bore MRI scanner. Radiofrequency (RF) transmission was achieved by using a quadratic transmit volumetric coil of 72 mm in diameter (Bruker T11232V3), and RF reception was achieved with a surface coil (Bruker T11657V3) ∼20 mm in diameter. CEST MRI was performed before and after i.v. injection of 200 µL Dex10 (10 kDa) saline solution (750 mg/kg body weight) according to the imaging scheme shown in Fig .1. The following parameters were used10,11: B1=1.8 µT, Tsat=3 s, and Δω=-3 to +3 ppm with a step size of 0.2 ppm. MTRasym=(S-Δω – S+Δω)/S0 was computed after B0 correction using the WASSR method. ΔMTRasym (1 ppm) at each time point was calculated by MTRasym (t)- MTRasym (pre).
RESULTS AND DISCUSSION
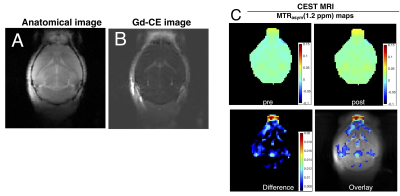
1. Size-variable BBB disruption in MS can be detected by fluorescent dextran-tracers of different sizes: Figure 2 shows that dextrans of smaller sizes (e.g., 3 kDa) penetrated the brain parenchyma deeper than larger sizes (e.g., 40 kDa), demonstrating the feasibility of using dextrans with different sizes as multi-scale imaging agents to probe the leakage size of BBB disruption.2. Dex-enhanced CEST MRI of BBB disruption in the EAE mouse model: Figure 3 shows the MR images of a representative mouse with a clinical disability score of 1.5, in which BBB disruption was visualized by Gd-enhanced MRI (Figure 3B). Dex-enhanced CEST MRI showed substantial contrast enhancement in corresponding brain regions (Figure 3C). Interestingly, while the size of Dex (10 kDa) is larger than the size of Gd-DOTA (559 Da), the area showing enhanced Dex10-CEST signals is slightly larger than that of Gd-enhancement. While the possible cause for this phenomenon is still under investigation, the data reveals that the other properties of imaging probes, such as shape, conformation, and surface charge, may also play an important role in BBB-permeation and caution has to be taken when interpreting imaging data acquired using different tracers.
CONCLUSION
We have established an MRI CEST imaging protocol for assessing the biodistribution of dextrans in the brains of EAE mice. Future studies using dextrans of different sizes are warranted to establish a CEST MRI protocol for assessing the size-dependent BBB disruption.Acknowledgements
No acknowledgement found.References
1. Wolfgang B, Andreas B, Herbert K, Yvonne B, Michael S, Hans L. Inflammatory central nervous system demyelination: Correlation of magnetic resonance imaging findings with lesion pathology. Annals of Neurology 1997;42(5):783-793.
2. Kermode AG, Tofts PS, Thompson AJ, MacManus DG, Rudge P, Kendall BE, Kingsley DP, Moseley IF, du Boulay EP, McDonald WI. Heterogeneity of blood-brain barrier changes in multiple sclerosis: an MRI study with gadolinium-DTPA enhancement. Neurology 1990;40(2):229-235.
3. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011;12(12):723-738.
4. Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab 2013;33(10):1500-1513.
5. Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 1991;17(2):357-367.
6. Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020;581(7806):71-76.
7. Lin Z, Li Y, Su P, Mao D, Wei Z, Pillai JJ, Moghekar A, van Osch M, Ge Y, Lu H. Non-contrast MR imaging of blood-brain barrier permeability to water. Magn Reson Med 2018;80(4):1507-1520.
8. Shao X, Ma SJ, Casey M, D'Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn Reson Med 2019;81(5):3065-3079.
9. Thomas AM, Yang E, Smith MD, Chu C, Calabresi PA, Glunde K, van Zijl PCM, Bulte JWM. CEST MRI and MALDI imaging reveal metabolic alterations in the cervical lymph nodes of EAE mice. J Neuroinflammation 2022;19(1):130.
10. Han Z, Chen C, Xu X, Bai R, Staedtke V, Huang J, Chan KWY, Xu J, Kamson DO, Wen Z, Knutsson L, van Zijl PCM, Liu G. Dynamic contrast-enhanced CEST MRI using a low molecular weight dextran. NMR Biomed 2022;35(3):e4649.
11. Li Y, Qiao Y, Chen H, Bai R, Staedtke V, Han Z, Xu J, Chan KWY, Yadav N, Bulte JWM, Zhou S, van Zijl PCM, Liu G. Characterization of tumor vascular permeability using natural dextrans and CEST MRI. Magn Reson Med 2018;79(2):1001-1009.
Figures