2616
Dual-Task Gait Predicts Changes in Choline in the Primary Motor Cortex of Older Individuals with Mild Cognitive Impairment1Neuroscience Department, Western University, London, ON, Canada, 2Gait and Brain Lab, Parkwood Institute and Lawson Health Research Institute, London, ON, Canada, 3Department of Medical Biophysics and Robarts Research Institute, Schulich School of Medicine and Dentistry, London, ON, Canada
Synopsis
Keywords: Dementia, Spectroscopy, Neuroinflammation, Dementia, Aging, Brain, Degenerative, Dementia, Neurodegeneration
The dual-task cost on walking speed (DTC) can predict the progression of dementia in people with mild cognitive impairment (MCI). In MCI, levels of choline in the primary motor cortex, an MRS metabolite associated with inflammation,Background
Mild cognitive impairment (MCI) is a transitional state between normal cognition and dementia, affecting nearly 12% -18% of people over the age of 601. A hallmark characteristic of the disease is the presence of increased inflammation in the brain, gait impairment and a higher risk of falls compared with cognitively preserved older adults. A previous study demonstrated that high levels of choline, a metabolite indicative of inflammation detected using magnetic resonance spectroscopy (MRS) in the primary motor cortex was negatively correlated with walking speed when MCI subjects were asked to simultaneously perform a cognitive task2. Attentional networks may compensate for damage to walking-related brain circuitry that may occur in people with MCI. Thus, overloading the attentional networks during a “dual-task paradigm” (DTP) is recognized as an effective assessment for MCI severity, as the level of gait impairment (dual-task cost) during the task is reflective of the degree of neurodegeneration3. The aforementioned study2 was cross-sectional, and therefore it is unknown whether the degree of gait impairment is associated with a change in choline over time. Thus, the purpose of this study was to better understand the relationship between choline concentration in the motor cortex and DTP gait speed by assessing whether gait impairment detected by a DTP can predict the change in choline over time.Hypothesis
We hypothesized that greater dual-task gait cost in speed at baseline would be associated with an increase in primary motor cortex choline concentrations after 6 months.Methods
110 participants with MCI were initially recruited through the Synchronized Exercises for Remedies in Gait and Cognition (SYNERGIC) study, a multi-site, longitudinal, randomized clinical trial. Due to attrition, MRS and gait data at baseline (T0) and after 6 months (T6) were obtained and analyzed from 65 participants [Age: mean = 74, SD = 6 years; Females: N = 33 (50.7%)]. Choline concentration in the primary motor cortex was measured by 1H magnetic resonance spectroscopy (TE/TR=135 ms/2000 ms, 8 cm3 voxel, 128 acquisitions) collected using 3T MRI and processed to obtain absolute metabolite levels (referenced to 8 unsuppressed water acquisitions) using custom software (fitMAN4, BARSTOOL5) developed and validated in our laboratory. Participants' gait speeds were recorded during usual or single-task and dual-task conditions at T0 using an electronic mat (Zeno walkway-Protokinetics). For the dual-task condition, participants were asked to ambulate while naming as many animals as possible out loud. The dual-task cost percentage (DTC) or the percentage change in gait speed from single to dual-task condition, was calculated by subtracting the single-task speed from the dual-task speed, and dividing by the single-task speed. A partial Pearson correlation controlling for age, sex, years of education, and MoCA scores was conducted to assess the relationship between T0 DTC in gait speed (%) and the percentage change in choline concentration (%) from T0 to T6. The alpha- was set to p < .05.Results
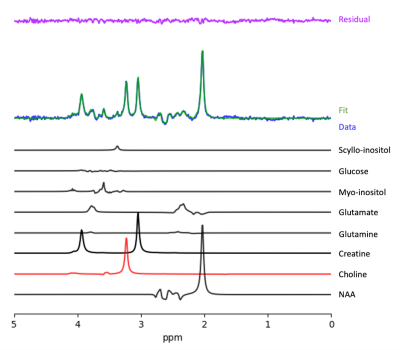
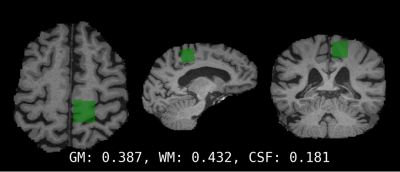
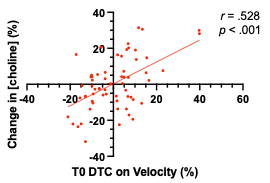
Data were successfully acquired and fitted (Figure 1) at both time points from the motor cortex (Figure 2) in 65 participants. Figure 3 shows a significant positive correlation between the DTC on gait speed at baseline, and the percent change in choline after 6 months in subjects with MCI (r= .528, p<.001, CI = .320 to .687).Discussion
These results suggest that individuals with MCI who showed greater reductions in gait speed while dual-tasking may have had increased primary motor cortex inflammation 6-months later. Although previous work has shown significant correlations between choline levels in the primary motor cortex and dual-task gait speed2, it is unclear whether inflammation is a contributor to slower dual-task gait speed. Volume of the primary motor cortex has been positively correlated to dual-task speed, and negatively correlated with choline levels in the motor cortex2. Furthermore, neurodegeneration due to pathogenic mechanisms of dementia has been known to ramp up inflammation in MCI6. It is possible that reductions in motor cortex volume may be the cause of impairment in dual-task gait speed, and that inflammation is a future by-product predicted to scale with the severity of neurodegeneration. This explanation is consistent with a previous study, where higher DTC in gait speed was associated with a greater risk of progressing to dementia7.Conclusion
Our work reinforces the relationship between changes in inflammation in the primary motor cortex and dual-task cost in gait speed. Future work should investigate whether these changes in choline measured using MRS persist beyond 6 months and whether changes in the volume of the primary motor cortex are also associated with DTP performance in MCI.Acknowledgements
No acknowledgement found.References
1. 2022 Alzheimer’s disease facts and figures. (2022). Alzheimer’s & Dementia, 18(4), 700–789. https://doi.org/10.1002/alz.12638
2. Annweiler, C., Beauchet, O., Bartha, R., Wells, J. L., Borrie, M. J., Hachinski, V., & Montero-Odasso, M. (2013). Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain: a journal of neurology, 136(Pt 3), 859–871. https://doi.org/10.1093/brain/aws373
3. Montero-Odasso, M., Speechley, M., Muir-Hunter, S. W., Sarquis-Adamson, Y., Sposato, L. A., Hachinski, V., Borrie, M., Wells, J., Black, A., Sejdić, E., Bherer, L., Chertkow, H., & Canadian Gait and Cognition Network (2018). Motor and Cognitive Trajectories Before Dementia: Results from Gait and Brain Study. Journal of the American Geriatrics Society, 66(9), 1676–1683. https://doi.org/10.1111/jgs.15341
4. Bartha R, Drost DJ, Williamson PC. Factors affecting the quantification of short echo in vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed 1999; 12:205-216
5. Wong D, Schranz A, Bartha R. Optimized in vivo brain glutamate measurement using long-echo time semi-LASER at 7T. NMR in Biomed. 2018:e4002. https://doi.org/10.1002/nbm.4002.
6. Ismail, Parbo, P., Madsen, L. S., Hansen, A. K., Hansen, K. V., Schaldemose, J. L., Kjeldsen, P. L., Stokholm, M. G., Gottrup, H., Eskildsen, S. F., & Brooks, D. J. (2020). The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. Journal of Neuroinflammation, 17(1), 151–151. https://doi.org/10.1186/s12974-020-01820-6
7. Montero-Odasso, Sarquis-Adamson, Y., Speechley, M., Borrie, M. J., Hachinski, V. C., Wells, J., Riccio, P. M., Schapira, M., Sejdic, E., Camicioli, R. M., Bartha, R., McIlroy, W. E., & Muir-Hunter, S. (2017). Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurology, 74(7), 857–865. https://doi.org/10.1001/jamaneurol.2017.0643
Figures