2615
Change in thalamic cerebrospinal fluid fraction over the adult lifespan and correlation with TSPO PET imaging in multiple sclerosis patients1Weill Cornell Medicine, New York, NY, United States, 2Howard University, Washington, DC, United States, 3University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Multiple Sclerosis, Neurofluids
We applied FAST-T2 multi-component T2 relaxometry to 200 MS patients and 66 healthy controls and found that the thalamic cerebrospinal fluid fraction (CSFF) increases with age and follows a different trajectory in MS. In 13 MS patients, we also found a strong correlation between CSFF and [11C]PK11195 uptake on PET in the thalamus and putamen, suggesting a connection between glymphatic dysfunction and microglial inflammation in the MS brain.INTRODUCTION
Multiple sclerosis (MS) is a neuroinflammatory disorder characterized by focal demyelination and axonal injury in WM and chronic progressive neurodegeneration in GM1. Recently, impairment in the glymphatic perivascular flow of cerebrospinal fluid (CSF)2 has been implicated as a potential pathological mechanism underpinning reduced waste clearance, tissue damage and clinical disability in the MS brain3. Increased CSF fraction (CSFF) on the microscopic level is a potential early indicator of glymphatic clearance dysfunction and can be mapped efficiently using advanced multi-echo T2 relaxometry sequences4 such as Fast Acquisition with Spiral Trajectory and adiabatic T2prep (FAST-T2)5. This approach can provide within each imaging voxel the fraction of highly mobile CSF occupying the perivascular space and that of the much less mobile water in the myelin sheath and the intra/extracellular space by utilizing differences in their T2 spectra6. We hypothesized that chronic inflammation in MS will have an adverse effect on the brain glymphatic clearance and correspondingly aimed to 1) to establish the difference in age-related CSFF trajectory between MS patients and healthy controls (HCs) over the adult lifespan, and 2) to study the relationship between MRI-derived CSFF and translocator protein (TSPO) PET, an imaging marker of microglial activation.METHODS
Study cohorts. For the age-related CSFF change study, a total of 200 MS patients (age range 22.3-79.5y, 145 women (72.5%), 55 men (27.5%), all RRMS, median EDSS=2) and 66 HCs (age range 22.4-79.8y, 40 women (60.6%), 26 men (39.4%)) who had FAST-T2 MRI scan were included. For PET-MRI correlation study, thirteen MS patients (9 women, 4 men, age 44.5y±13.4, 8 RRMS, 3 PPMS, 2 SPMS) who had both [11C]PK11195 TSPO PET and FAST-T2 MRI scans within a short interval (median 2 days) were included. Patients with active inflammatory disease as evidenced by new Gd-enhancing lesions were excluded.MRI acquisition. The 3T MRI protocol (Siemens and GE scanners) included a 4 min FAST-T2 sequence (1x1x5 mm3 voxel size) with geometric echo spacing for multi-component T2 relaxometry5 in addition to the conventional T1W, T2W and FLAIR sequences. A spatially regularized three-pool non-linear least squares algorithm using the L-BFGS solver was used to compute myelin water fraction, intra/extracellular water fraction, and CSFF maps from the six-echo T2 decay data5. The lower and upper T2 bounds for each of the three water pools (in msec) were set to [5 20], [20 200], and [200 2000], respectively. Freesurfer v6 recon-all command was applied to T1W/T2W images to obtain brain segmentation7. CSFF maps were aligned to Freesurfer T1W image using FMRIB’s Linear Image Registration Tool8. To reduce contamination from CSF occupying the brain ventricles and the subarachnoid space due to partial voluming, the segmented brain regions of interest were first eroded by 1mm and then further eroded to be at least 1 mm in-plane and 5 mm through-plane from the boundary of these CSF-filled spaces.
[11C]PK11195 PET acquisition. PET images were acquired on a lutetium oxyorthosilicate (LSO) time-of-flight PET/CT scanner (Siemens/CTI) and reconstructed using an iterative-plus-time of flight list-mode reconstruction algorithm provided by the manufacturer using OSEM methods. Tissue concentrations were estimated by reconstructing data into 22 frames for a total scan time of 60 min. Regional distribution volume ratios (DVRs) of radioligand uptake were calculated using Logan graphical model9. A computational supervised clustering methodology (SuperPK) was used to extract a reference curve on TSPO PET. PET images were aligned to Freesurfer T1W image using PMOD software.
Statistical analysis. Based on visual inspection of thalamic CSFF vs. age data, a linear regression model was used to regress CSFF on age (with indicator for >50y group) adjusting for sex, disease status (MS or HC), and thalamic volume (normalized to skull size) with a three-way interaction among disease status, age, and age indicator. Linear correlation was obtained between mean TSPO PET DVR and MRI CSFF values in the thalamus and putamen, two large subcortical GM regions that have been implicated in MS in recent TSPO PET studies10,11.
RESULTS
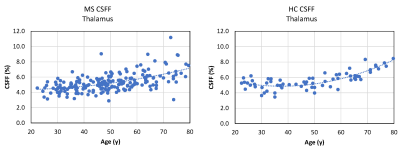
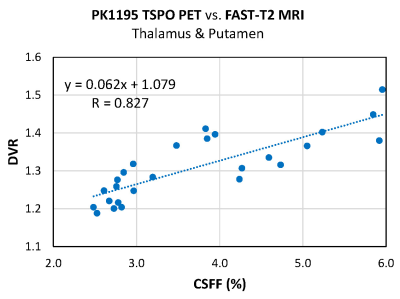
In the linear model with CSFF as an outcome, we found a significant association between thalamic CSFF and >50y age indicator (p=0.042) and that having MS is likely to alter the relationship between thalamic CSFF and age before the age of 50 (p=0.072). The other statistically significant variables in the model were MS disease status (p=0.006) and normalized thalamic volume (p<0.001). Figure 1 shows age-related CSFF changes in MS and HC subjects, showing a steady increase of thalamic CSFF with age over the whole adult lifespan in MS patients, while the rise in CSFF only becomes noteworthy after the age of 50 in HCs. We found a strong linear correlation between TSPO PET DVR measurements and corresponding MRI-derived CSFF in the thalamus and putamen (Fig.2, R=0.827, p=1.9e-7).DISCUSSION
We found different trends in CSFF increase with age in MS vs. HC, which underlies the adverse impact of ongoing neuroinflammation and neurodegeneration on the MS brain beyond that observed in normal aging. Our results also provided the initial evidence that the MRI-derived CSFF measures in subcortical GM correlate well with [11C]PK11195 uptake by TSPO PET, suggesting a link between glial inflammation and reduced glymphatic clearance in these areas.Acknowledgements
No acknowledgement found.References
1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. The New England Journal of Medicine 2018;378:169-80.
2. Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain 2022;145:2785-95.
3. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018;17:1016-1024. 4. Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: A technical review. Magn Reson Med 2015;73:70-81.
5. Nguyen TD, Deh K, Monohan E, Pandya S, Spincemaille P, Raj A, Wang Y, Gauthier SA. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med 2016;76:456-65.
6. MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Mädler B. Insights into brain microstructure from the T2 distribution. Magn Reson Imaging 2006;24:515-25.
7. Fischl B. FreeSurfer. Neuroimage 2012;62:774-81.
8. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17(2):825-41.
9. Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834-40.
10. Kang Y, Pandya S, Zinger N, Michaelson N, Gauthier SA. Longitudinal change in TSPO PET imaging in progressive multiple sclerosis. Ann Clin Transl Neurol 2021;8:1755-9.
11. Misin O, Matilainen M, Nylund M, Honkonen E, Rissanen E, Sucksdorff M, Airas L. Innate Immune Cell-Related Pathology in the Thalamus Signals a Risk for Disability Progression in Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2022;9:e1182.
Figures