2613
Microstructural alterations in gray and white matter integrity in migraine with aura1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Neurology, New York University Grossman School of Medicine, New York, NY, United States, 3Division of Neuroradiology, Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: Gray Matter, Neuroinflammation
Migraine is one of the most common neurological disorders. Thirty percent of migraine patients develop transient neurological symptoms, the so-called migraine aura. Migraineurs, particularly those with aura, show an increased incidence of white matter lesions (WML). To improve our understanding of WML in migraineurs, we utilized Soma and Neurite Density Image (SANDI) as an advanced diffusion magnetic resonance imaging signal model to assess in-vivo microstructure of gray matter and white matter. We found evidence for occult changes in microstructural gray and white matter integrity that point to a common underlying mechanism driving cellular inflammation and neuronal injury in migraine.Introduction
Migraine is one of the most common neurological disorders, characterized by unilateral headaches that last for 4-72 hours. Thirty percent of migraine patients develop transient neurological symptoms in the setting of an attack, the so-called migraine aura [1]. Neuroimaging studies have identified a two- to four-fold increased incidence of white matter hyperintensities suggestive of white matter lesions (WML) in migraineurs, particularly in patients with aura [2-7]. A variety of advanced MRI techniques have been applied to improve our understanding of the microstructural substrate of WMLs in migraineurs [2, 8]. Here, we utilize Soma and Neurite Density Image (SANDI) as an advanced diffusion magnetic resonance imaging signal model to characterize in vivo microstructure of gray matter (GM) and white matter (WM) in migraine patients with aura.Methods
Subjects16 migraine patients with aura and 16 age- and sex-matched healthy controls (HC) participated in this IRB-approved study. Demographic and clinical features are shown in Table 1.
MRI data acquisition
All participants underwent MR imaging on a 3T Connectome scanner (MAGNETOM Connectom, Siemens Healthcare, Erlangen, Germany) equipped with a maximum gradient strength of 300 mT/m. A diffusion multi-shell acquisition protocol consisting of 19 msec diffusion time and 8 b-values ranging from 0.05 - 6 um2/ms (32 directions for b-values < 2.3 um2/ms, 64 directions for b-values 2.4 > um2/ms) was implemented in all subjects [9]. Structural T1W 3D MPRAGE (TR/TE = TR/TE = 2530/[1.15, 3.03, 4.89, 6.75], inversion time (TI) =1100 msec, 1×1×1 mm3 voxels, r = 2), and DTI (TE/TR = 3600/77 msec, 2×2×2 mm3 voxels, r = 2) of the whole brain and FLAIR images [TE/TR/TI=389/5000/1800 msec, 0.9×0.9×0.9 mm3 voxels, r = 2] were obtained.
MRI data analysis
We used the MGH-USC Human Connectome project's data preprocessing pipeline [10], including the Freesurfer [11] (http://surfer.nmr.mgh.harvard.edu) and FMRIB Software Library [12] (https://fsl.fmrib.ox.ac.uk) toolboxes for analysis.
SANDI metrics of cell body signal fraction, radius signal fraction, intra-neurite and extra-neurite maps were calculated [13] using the SANDI-AMICO toolbox on the DWI images as presented in Figure 1. SANDI microstructural metrics were compared in WM and GM regions between migraine patients and HC and correlated with age, cortical thickness, and memory scores.
Statistical analysis
A two-sample t-test was used to find differences between migraine patients and healthy controls for demographic, clinical, and MRI-based variables. The correlations between the clinical variables, demographic features, and SANDI metrics were assessed using Pearson's correlation coefficient. P-values < 0.05 were considered significant and were Bonferroni corrected for multiple comparisons.
Results
As shown in Table 2, both WM and GM apparent soma radius and intra-neurite signal fraction maps revealed a reduction in migraine patients compared to HC (p < 0.0001, p = 0.015, p = 0.28, and p = 0.0002, respectively). WM cell body signal fraction was increased in patients compared to HC (p = 0.001).Regarding the ROI-based analysis of SANDI, apparent soma radius and intra-neurite signal fraction of migraine patients revealed a significant reduction in the thalamus, putamen, amygdala, accumbens bilaterally, and left caudate and pallidum when compared to HC (p<0.04). Intra-neurite signal fraction of the hippocampus decreased significantly in patients compared to HC (p <0.0001). In patients, apparent soma radius revealed a significant decrease in the caudate compared to healthy controls. Additionally, compared to HC, patients' cell body signal fraction was increased significantly in the thalamus, putamen, hippocampus, and amygdala (p<0.04).
Moreover, intra-neurite signal fraction, apparent soma radius, and cell body signal fraction of white matter lesions increased significantly compared to HC and NAWM of the same subjects (p <0.04) while extra-neurite signal fraction increased significantly (p <0.0001).Healthy controls revealed a positive correlation between cell body signal fraction and cortical thickness (r = 0.7 and p = 0.003) and a negative correlation between intra-neurite signal fraction and cortical thickness in GM, while this relation was not observed in migraine patients. WM and GM volume was not associated with any of the SANDI metrics in the migraine patients. There was no significant relationship between SANDI metrics in WM or GM and memory scores in the migraine patients.
Discussion
We found significantly decreased intra-neurite signal fraction, increased soma signal fraction, and decreased soma radius in the WM of migraine patients compared to HC. These trends were replicated in several deep gray structures that have been previously reported to show volume loss in migraine [2], notably the thalamus, putamen, hippocampus, and amygdala. These findings suggest a common underlying mechanism driving microstructural changes in white matter and gray matter of migraine patients. The repetitive occurrence of the electrophysiologic event spreading depolarization during migraine attacks may be a key mechanism, causing cumulative cellular injury and microstructural gray and white matter changes through episodic cerebral hypoperfusion and neuroinflammation [14]. Future research will focus on validating these findings in a larger cohort of migraine patients and correlating these results with findings in animal models, to further characterize the underlying microstructural substrate of injury in migraine.Conclusion
SANDI offers insight into microstructural alterations in gray and white matter integrity in migraine patients with aura, suggesting a common underlying mechanism driving observed increases in cellular density and neurite loss.Acknowledgements
This work was supported by funding from the National Institutes of Health under grant numbers R01NS118187 and P41EB030006 and the Ralph Schlaeger award.References
[1] Ziegler, Dewey K., and Ruth S. Hassanein. “Specific headache phenomena: their frequency and coincidence.” Headache: The Journal of Head and Face Pain, vol. 30, no. 3, 1990, pp. 152–156., doi:10.1111/j.1526-4610.1990.hed3003152.x.
[2] Ashina, Sait, et al. “Structural and functional brain changes in migraine.” Pain and Therapy, vol. 10, no. 1, 2021, pp. 211–223., doi:10.1007/s40122-021-00240-5.
[3] Bashir, Asma, , et al. "Migraine and structural changes in the brain". Neurology, vol. 81, no. 14, October 1, 2013, pp. 1260-1268. doi: 10.1212/WNL.0b013e3182a6cb32.
[4] Swartz, Richard H., and Ralph Z. Kern. “Migraine is associated with magnetic resonance imaging white matter abnormalities.” Archives of Neurology, vol. 61, no. 9, 2004, p. 1366., doi:10.1001/archneur.61.9.1366.
[5] Zhang, Quan, et al. “White matter lesion burden in migraine with aura may be associated with reduced cerebral blood flow.” Cephalalgia, vol. 37, no. 6, 2016, pp. 517–524., doi:10.1177/0333102416649760.
[6] Hamedani, Ali, G., et al. "Migraine and white matter hyperintensities". Neurology, vol. 81, no. 15, October 8, 2013, pp. 1308-1313. doi: 10.1212/WNL.0b013e3182a8235b.
[7] Gaist, David, et al. “Migraine with aura and risk of silent brain infarcts and white matter hyperintensities: An MRI study.” Brain, vol. 139, no. 7, 2016, pp. 2015–2023., doi:10.1093/brain/aww099.
[8] Arca, Karissa N., et al. “Narrative review of neuroimaging in migraine with aura.” Headache: The Journal of Head and Face Pain, vol. 61, no. 9, 2021, pp. 1324–1333., doi:10.1111/head.14191.
[9] Huang SY, Tian Q, Fan Q, Witzel T, Wichtmann B, McNab JA, Bireley JD, Machado N, Klawiter EC, Mekkaoui C, Wald LL, Nummenmaa A. High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain. Brain Structure and Function. 2020 May;225(4):1277-1291. doi: 10.1007/s00429-019-01961-2.
[10] Q. Fan et al., “MGH-USC Human Connectome Project datasets with ultra-high b-value diffusion MRI.,” Neuroimage, vol. 124, no. Pt B, pp. 1108–1114, Jan. 2016, doi: 10.1016/j.neuroimage.2015.08.075.
[11] B. Fischl, “FreeSurfer,” NeuroImage. 2012, doi: 10.1016/j.neuroimage.2012.01.021.
[12] S. M. Smith et al., “Advances in functional and structural MR image analysis and implementation as FSL.,” Neuroimage, vol. 23 Suppl 1, pp. S208-19, 2004, doi: 10.1016/j.neuroimage.2004.07.051.
[13] M. Palombo et al., “SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI,” Neuroimage, vol. 215, pp. 116835, 2020, doi: https://doi.org/10.1016/j.neuroimage.2020.116835.
[14] Eikermann-Haerter, Katharina, and Susie Y. Huang. “White matter lesions in migraine.” The American Journal of Pathology, vol. 191, no. 11, 2021, pp. 1955–1962., doi:10.1016/j.ajpath.2021.02.007.
Figures
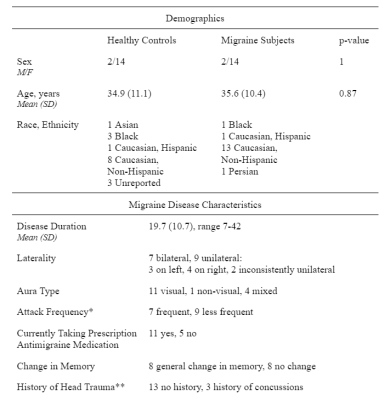
Table 1. Demographic and clinical features of study participants
* Frequent attacks defined as a minimum of 10 per month
** No recent head trauma nor coinciding with initial migraine onset
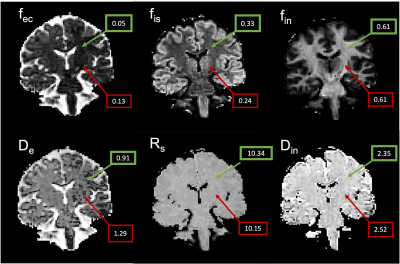
Figure 1. SANDI parameter maps derived from DWI data in a representative migraine patient.
fec = extra-cellular signal fraction; fis = intra-soma signal fraction; fin = intra-neurite signal fraction; Rs = apparent soma radius. Green boxes indicate representative values in cerebral white matter; red boxes indicate representative values in the accumbens (gray matter).
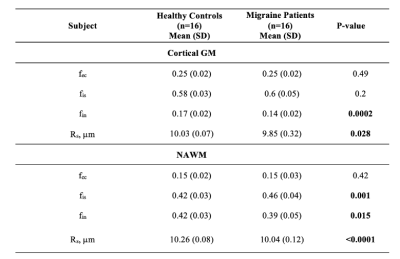
Table 2. Migraine patients and healthy controls SANDI metrics values
fec = extra-cellular signal fraction; fis = intra-soma signal fraction; fin = intra-neurite signal fraction; Rs = apparent soma radius; GM = gray matter; NAWM = normal-appearing white matter.