2612
White matter tracts of MS patients with progression independent of relapse activity show DTI-alterations compared to clinically stable patients
Mario Ocampo-Pineda1,2,3, Alessandro Cagol1,2,3, Muhamed Barakovic1,2,3, Po-Jui Lu1,2,3, Jannis Müller1,2,3, Sabine Schaedelin4, Pascal Benkert4, Matthias Weigel1,2,3,5, Lester Melie-Garcia1,2,3, Jens Kuhle2,3, Ludwig Kappos1,2,3, and Cristina Granziera1,2,3
1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, Faculty of Medicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2Department of Neurology, University Hospital Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland, 4Clinical Trial Unit, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, 5Division of Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland
1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, Faculty of Medicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2Department of Neurology, University Hospital Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland, 4Clinical Trial Unit, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, 5Division of Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland
Synopsis
Keywords: White Matter, Diffusion Tensor Imaging
Progression independent of relapse activity (PIRA) has been described in patients with multiple sclerosis (MS) even in the earliest disease stages. Patients with PIRA show increased atrophy rates in multiple brain regions compared to stable patients. Here, we investigated whether patients with PIRA exhibit loss of integrity in WM tracts compared to stable patients. We studied 62 RRMS patients, 27 PIRA and 35 stable patients using a clinical DW-MRI protocol. Our results showed that PIRA patients present smaller FA values in areas of corpus callosum and along corticosprinal tract. These differences suggest neurodegeneration in major WM tracts of PIRA patients.Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system, which is characterized by inflammatory, demyelinating, and neurodegenerative processes1. Disability in patients with MS may result from neuroinflammatory events occurring in clinical relapses and during radiological activity2, but also through mechanisms of progression that are independent of relapse activity (PIRA). It has been shown that patients with PIRA present increased atrophy rates in multiple brain regions compared to stable patients3. Whether the degeneration of major white matter (WM) tracts plays a role in the development of PIRA, it is currently unknown.Diffusion Tensor Imaging (DTI) uses a second-order symmetric and positive-definite matrix (tensor) to quantify the magnitude and direction of water diffusion in biological tissues4,5. DTI-derived metrics, like fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD), seem to provide a better specificity to demyelination and axonal injury6,7 than conventional MRI measures.
Here, we investigate whether patients with PIRA exhibit loss of integrity in normal-appearing white matter (NAWM) of WM tracts compared to stable patients with MS employing DTI-derived metrics from a clinical protocol.
Methods
From 309 patients in the Swiss MS Cohort Study, we studied 62 patients with relapsing remitting multiple sclerosis (RRMS) with a median follow-up (FU) of 3.2 years (IQR:2.0-4.9), patients with PIRA had a median FU of 4.0 years (IQR:2.0 to 5.0) and stable patients of 3.0 (IQR:1.5 to 4.3). We identify patients with PIRA that presented an expanded disability status scale (EDSS) increase of ⩾1.5 if baseline EDSS=0, ⩾1.0 if 1.0-5.5, ⩾0.5 if >5.5 confirmed after ⩾ 6 months, and relapse-free over the entire FU8. All patients underwent MRI including MPRAGE (1x1x1mm3) and single shell DW-MRI (1.8x1.8x1.8mm3) with 20 diffusion directions, bmax-value=1000 s/mm2 and 10 non-diffusion weighted images (b-value=0 s/mm2). Some subjects had multiple FU acquisitions.DW-MRI were preprocessed using tools from FSL9 and MRtrix310: denoising, correction of eddy currents and artifacts, and bias field correction. We computed the diffusion tensor to generate maps of DTI-derived metrics FA, AD, MD, and RD. We also performed constrained spherical deconvolution to compute fiber orientation distribution functions. With these, we performed WM tract reconstruction using TractSeg11 and segmented the bundles Corticospinal Tract (CST, left and right), and the Corpus Callosum (CC, divided into 7 segments). We performed a voxel-based and a Tractometry12 analysis, in the last one the bundles were divided into segments used as regions of interest.
For the voxel-based analysis, we registered the subjects to the MNI152 template13 to perform voxel-based statistics using age and gender as covariates and excluding the voxels with lesions. After performing family-wise error correction and a threshold-free cluster enhancement14, we applied a statistical threshold of p<0.05. To perform the Tractomety analysis, we modified the implementation to exclude the voxels labeled as lesions to compute the mean values of the voxels in the segments of the bundles. We compared the segments generated with Tractometry for patients with PIRA and stable, adjusting by age and sex. Then a permutation-based multiple comparison correction was performed15,16.
Results
We compared 27 PIRA (40 scans) with 35 stable patients (57 scans). In the voxel-based analysis, patients with PIRA showed smaller FA values in the region of corpus callosum and along the CST, whereas MD, AD and RD were increased for stable patients in areas of partial volume with cortical gray matter (MD, RD, AD) and cerebrospinal fluid (AD). Figure 1 shows the areas of significant differences in red and yellow for FA, MD, RD, and AD in the CC and CST, and Table 1 shows the average and standard deviation of the metrics in the selected areas (corrected p-value<0.05).Figures 2 and 3 show the mean FA and RD respectively along the bundles for both groups. It is possible to see in the CST_rigth that the mean FA of stable patients is higher than the one in patients with PIRA, which is in agreement with the results of the voxel-based analysis. In contrast, RD is higher for patients with PIRA in CC_1 and CC_2, which differs from the previous analysis. It has to be noted, that the results of this analysis were not statistically significant in any segment of the bundles analyzed.
Discussion
Patients experiencing PIRA showed increased microstructural damage suggesting axonal degeneration in the CST and CC, especially in the brainstem, internal capsule, and corona radiata. On the other hand, MD, RD, and AD showed changes in regions of partial volume.Conclusions
We showed evidence of neurodegeneration in major white matter tracts in patients with clinical worsening independent of inflammatory activity. These differences suggest that Wallerian degeneration processes17 contribute to the development of disability in those patients.Acknowledgements
Swiss Multiple Sclerosis Cohort studyReferences
- Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration: recent insights from MS pathology. Biochim Biophys Acta. 2011;1812 (2):275-282. doi:10.1016/j.bbadis.2010.07.007.
- Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61(11):1528-1532. doi:10.1212/01.WNL.0000096175.39831.21.
- Cagol A, Schaedelin S, Barakovic M, et al. Association of Brain Atrophy With Disease Progression Independent of Relapse Activity in Patients With Relapsing Multiple Sclerosis. JAMA Neurol. 2022;79(7):682–692.
- Basser, P. J., Mattiello, J., and LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophysical journal, 1994;66(1):259–267. S0006-3495(94)80775-1[PII].
- Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A., and Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology, 1996;201(3):637–648.PMID: 8939209.
- Sun, S. W., Liang, H. F., Le, T. Q., Armstrong, R. C., Cross, A. H., & Song, S. K. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage, 2006;32(3), 1195–1204. S1053-8119(06)00540-4.
- Kolasa, M, Hakulinen, U, Brander, A, et al. Diffusion tensor imaging and disability progression in multiple sclerosis: A 4-year follow-up study. Brain Behav. 2019; 9:e01194. https://doi.org/10.1002/brb3.1194
- Kappos L, Butzkueven H, Wiendl H, et al. Tysabri® Observational Program (TOP) Investigators. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24 (7):963-973. doi:10.1177/1352458517709619.
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M.. FSL. NeuroImage, 2012;62:782-90.
- Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019;202:116137.
- Wasserthal, P. Neher, K.H. Maier-Hein TractSeg—fast and accurate white matter tract segmentation Neuroimage, 183 (2018), pp. 239-253, 10.1016/j.neuroimage.2018.07.070
- Wasserthal, J., Maier-Hein, K.H., Neher, P.F. et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacol. 2020; 45, 1750–1757. https://doi.org/10.1038/s41386-020-0691-2.
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric Atlasing and Model Based Segmentation: An Application to the Hippocampus in Older Adults. In: ; 2006:58-66. doi:10.1007/11866763_8.
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference NeuroImage, 2009:44 (1), pp. 83-98
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLOS ONE 2012; 7(11): e49790. https://doi.org/10.1371/journal.pone.0049790.
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15: 1–25.
- Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging--evidence of Wallerian degeneration. J Neurol. 2003;250(3):287-292. doi:10.1007/s00415-003-0992-5
Figures
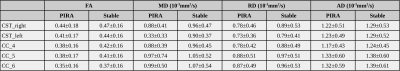
Table 1. Average and standard deviation of the diffusion tensor metrics in the voxels with statistically significant difference (corrected p-value<0.05). FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.
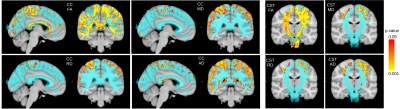
Figure 1. Statistically significant difference (corrected p-value<0.05) in voxels crossed by tracts part of Corpus Callosum (CC) and Corticospinal Tract (CST). Stable patients have greater diffusion tensor metrics than patients with PIRA. On blue are the mask of the tracts of CC and CST. FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.
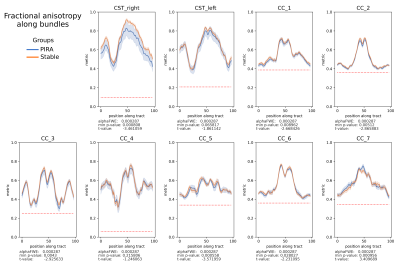
Figure 2. Mean value per group PIRA (blue) and Stable (orange), of the fractional anisotropy (FA) of 100 segments along the bundles Corticospinal Tract (CST) and the Corpus Callosum (CC) divided into seven parts. alphaFWE is the alpha value corrected for multiple positions per bundle and multiple bundles. min p-value is the minimal p-value of the segments. The red dotted line indicates all positions within the bundle where p-value<alphaFWE. FA describes the overall directionality of water diffusion.
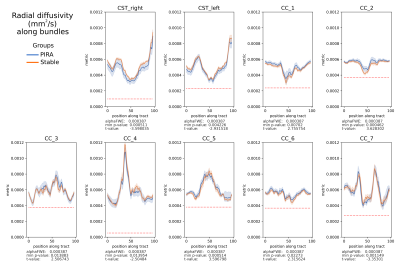
Figure 3. Mean value per group PIRA (blue) and Stable (orange), of radial diffusivity (RD) (mm2/s) of 100 segments along the bundles Corticospinal Tract (CST) and the Corpus Callosum (CC) divided into seven parts. alphaFWE is the alpha value corrected for multiple positions per bundle and multiple bundles. min p-value is the minimal p-value of the segments. The red dotted line indicates where p-value<alphaFWE. RD is the average diffusivity perpendicular to the main diffusion direction.
DOI: https://doi.org/10.58530/2023/2612