2609
Free water alterations of deep gray matters over 1-2 years in small vessel disease
Yawen Sun1, Hongjiang Wei2, and Yan Zhou1
1Department of Radiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
1Department of Radiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Synopsis
Keywords: Dementia, Diffusion/other diffusion imaging techniques, Free water mapping
This study was to evaluate the neuroinflammation-related characteristics of the deep gray matter using free-water mapping in small vessel disease over 1-2 years. Our results support the presence of elevated free water at the preclinical stage of small vessel disease, which remains persistent during the early course of the illness. The free water values changes might be the biomarkers for the mechanism of cognitive decline in the evolution of small vessel disease.Purpose
The aim of this study was to evaluate the neuroinflammation-related characteristics of the deep gray matter (DGM) using free-water (FW) mapping in small vessel disease (SVD) over 1-2 years.Methods
Sixty-seven SVD patients with no cognitive impairment (NCI) (the preclinical stage), 103 with mild cognitive impairment (VaMCI) (early clinical stage) and 21 healthy controls underwent brain MRI scans and neuropsychological evaluations at baseline. Of these, 28 VaMCI and 23 NCI participated in the follow-up study 1-2 years later. FW values within DGMs including the bilateral caudate nucleus, globus pallidus, putamen, substantia nigra, red nucleus, and dentate nucleus were calculated for each individual. In addition, FW values in the thalamus were calculated in the subregion, including anterior nuclei, the median nuclei, the lateral nuclei (LN), the pulvinar (Pul) and the internal medullary lamina (IML). Cross-sectional group comparisons were performed with one-way ANOVA and post hoc t-tests. In longitudinal comparison, temporal and cross-sectional differences were studied using mixed factorial ANOVA analysis and appropriate t-tests. Partial correlations were used to assess the relationship between the FW values changes (ΔFWfollowup-baseline/FWbaseline) and cognitive function changes (i.e., ΔMoCAfollowup-baseline/MoCAbaseline).Results
Significantly higher FW values (index of neuroinflammation) were found in DGMs at baseline in both VaMCI and NCI compared to healthy controls. In longitudinal comparison, the Group effect resulted in greater mean FW values in the left Pul, bilateral LN and bilateral IML of the thalamus for VaMCI compared with NCI. However, there were no significant effects for the FW values across Time or Group × Time interactions. Furthermore, we observed significant negative associations between the FW values changes in the left Pul and the right LN and MoCA scores changes in VaMCI group over 1-2 years.Discussion
FW mapping obtained from a bi-tensor diffusion MR imaging model was developed, which was established to explicitly estimate the fractional volume of freely diffusing water molecules within the voxel. It can provide extracellular FW changes, such as inflammation, as measured by the fractional volume of FW. It is well known that white matter lesions are considered the hallmark of SVD. Recently study in our group demonstrated that the FW values in the white matter are elevated in SVD, which indicated tissue neuroinflammation or edema. The increased FW was also the strongest predictor of cognitive function. However, the DGMs, such as the thalamus and basal ganglia, are also involved in the progression of SVD and contribute to cognitive impairment. Chronic global hypoperfusion is the prominent feature of SVD, which can cause a range of brain injuries. The DGMs, which mainly receive blood from deep penetrating arteries, such as lenticulostriate arteries, are particularly susceptible to hypoperfusion in SVD, with the progressive development of neurodegeneration accompanied by robust inflammation. There is evidence that inflammation is longitudinally associated with SVD progression, especially if the inflammatory response is sustained in the long term. Moreover, this inflammation process also contributes to the gradual iron deposition that may catalyze the formation of free-radical mediated damage, hence exacerbating neurodegeneration. Together, our results suggest inflammation in DGMs may play a critical role in SVD.Conclusion
Our results support the presence of elevated FW at the preclinical stage, which remains persistent during the early course of the illness. The FW values changes might be the biomarkers for the mechanism of cognitive decline in the evolution of SVD.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81901693), Shanghai "Rising Stars of Medical Talent" Youth Development Program, Youth Medical Talents-Medical Imaging Practitioner Program (Grant No. SHWRS(2020)_087).References
- Wardlaw JM, Smith C and Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483-497. DOI: 10.1016/S1474-4422(13)70060-7.
- Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology 2013; 12: 822-838. 2013/07/23. DOI: 10.1016/s1474-4422(13)70124-8.
- Low A, Mak E, Rowe JB, et al. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res Rev 2019; 53: 100916. 20190610. DOI: 10.1016/j.arr.2019.100916.
- Pasternak O, Sochen N, Gur Y, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009; 62: 717-730. 2009/07/23. DOI: 10.1002/mrm.22055.
- Mayer C, Nägele FL, Petersen M, et al. Free-water diffusion MRI detects structural alterations surrounding white matter hyperintensities in the early stage of cerebral small vessel disease. J Cereb Blood Flow Metab 2022: 271678x221093579. 20220411. DOI: 10.1177/0271678x221093579.
- Sun Y, Hu Y, Qiu Y, et al. Characterization of white matter over 1-2 years in small vessel disease using MR-based quantitative susceptibility mapping and free-water mapping. Front Aging Neurosci 2022; 14: 998051. 20220930. DOI: 10.3389/fnagi.2022.998051.
Figures
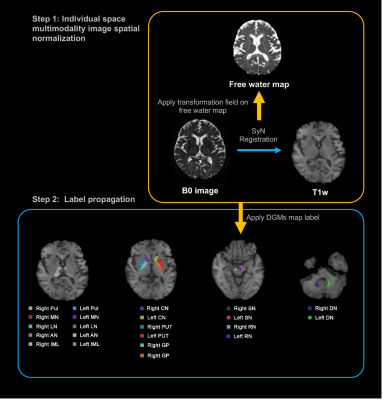
Illustration of a processing pipeline of image registration and generation of labels.
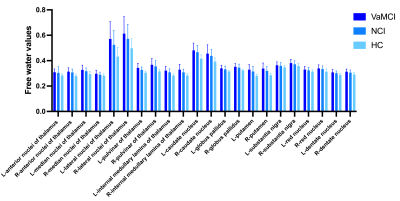
Free-water values in the deep gray matter among the three groups.
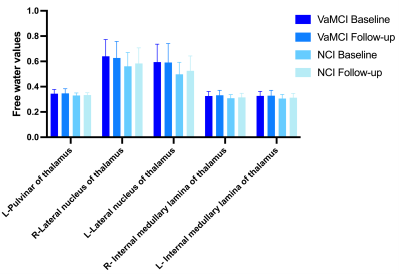
Free-water values in the deep gray matter between baseline and follow-up.
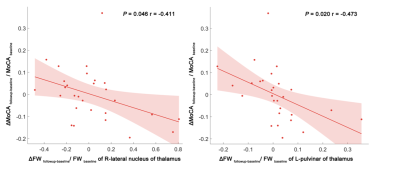
Scatter plots and linear regression illustrating the relationship between longitudinal free water changes and MoCA score changes. The dots represent the adjusted values after controlling age, gender, years of education, and mean follow-up time.
DOI: https://doi.org/10.58530/2023/2609