2607
Lumbar spine disc degeneration assessed by in vivo 3T multifrequency MR elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2BIH Biomedical Innovation Academy, BIH Charité Digital Clinician Scientist Program, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany, 3Radiology, Universitätsklinikum Augsburg, Augsburg, Germany, 4Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Keywords: Elastography, MSK
Despite the success in detecting intervertebral disc degeneration with high-resolution T2 sequences, conventional MRI is limited by large inter-reader variability as well as lacking stratification of clinical trials and their assessment of treatment responses. Several studies have shown the technical feasibility of MR elastography for the study of the intervertebral disc, but its clinical relevance remains unclear. This study shows an excellent diagnostic performance of high-resolution multifrequency MR elastography in the evaluation of lumbar spine intervertebral disc degeneration.
Introduction
Intervertebral disc (IVD) degeneration is one of the most common causes of low back pain and has been associated with several pathological conditions such as disc herniation or spinal stenosis 1. The causes of IVD degeneration include many factors such as mechanical stress, biochemical influences, age, smoking and genetics 2. Despite the success in detecting IVD degeneration with high-resolution T2 sequences, conventional MRI is limited by large inter-reader variability as well as lacking stratification of clinical trials and their assessment of treatment responses 3,4. Several studies have shown the technical feasibility of MR elastography (MRE) for the study of the IVD, but its clinical relevance remains unclear 5-10. Therefore, we aimed at investigating the diagnostic performance of MRE for the assessment of lumbar spine IVD degeneration.Methods
The study was approved by the local IRB and written informed consent was obtained from all subjects. 30 patients with low back pain and 30 volunteers without low back pain were studied in a 3T MRI scanner (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) with high resolution multifrequency MRE at 40, 50, 60 and 70 Hz and tomoelastography postprocessing to determine shear wave speed (SWS in m/s) and loss angle of the complex modulus (φ in rad), which represent stiffness and viscosity-related dispersion of stiffness over frequency, respectively 11,12. A board-certified musculoskeletal radiologist (D.K.) determined the Pfirrman score using conventional T2 sequences to grade IVD degeneration from I (no degeneration) to V (high degeneration) 3. The Pfirrman score is the best noninvasive diagnostic reference available and is based on a subjective assessment of T2 signal intensity, homogeneity and height of the disc 3. One lumbar IVD was examined in each subject.Results
Mean age and mean body mass index were 41±17 years and 23.9±3.7 kg/m2 (n=60, 25 women). Figure 1 shows a case of a patient with IVD degeneration and a healthy volunteer. Figure 2 shows boxplots of MRE parameters according to Pfirrman scores. Mean values of SWS and φ were as follows: Pfirrman score I (n=8), 2.62±0.45 m/s and 0.91±0.08 rad; Pfirrman score II (n=17), 2.45±0.46 m/s and 0.85±0.07 rad; Pfirrman score III (n=9), 2.13±0.34 m/s and 0.78±0.07 rad; Pfirrman score VI (n=21), 1.45±0.25 m/s and 0.62±0.09 rad; and Pfirrman score V (n=5), 1.04±0.09 m/s and 0.51±0.03 rad. Spearman correlation revealed a statistically significant strong negative correlation between MRE parameters and the Pfirrman score (SWS: r=-0.86, p<0.001; and φ: r=-0.87, p<0.001). Area under the receiver operating characteristic (AUC) analysis with 95% confidence interval showed a diagnostic performance for detecting Pfirrman score greater II/III/IV/V, respectively, as follows: for SWS: 0.85 (0.75-0.94)/0.93 (0.87-0.98)/0.995 (0.99-1.00)/0.96 (0.91-0.996); for φ 0.89 (0.80-0.98)/0.95 (0.90-0.99)/0.98 (0.95-1.00)/0.94 (0.88-0.98).Discussion
In this study, we have investigated the diagnostic performance of MRE for the assessment of lumbar spine IVD degeneration and have shown an excellent diagnostic performance for both SWS and φ using the MRI-based Pfirrman score as reference. Both stiffness and viscosity-related dispersion of stiffness over frequency decreased with the degeneration of the IVD. This decrease of viscoelastic properties could be associated with the degradation of extracellular matrix components such as collagen or glycosaminoglycans and decreased water content 1. Possibly, the characterization of viscoelastic properties may inform early treatment decisions for the mechanical regeneration of the IVD.In agreement with our results, Cortes et al. found in an ex vivo MRE study with cadaveric human lumbar spines that the shear modulus of the nucleus pulpous significantly decreased with degeneration 10. Likewise, Streitberger et al. demonstrated the feasibility of in vivo MRE in 16 asymptomatic volunteers (IVD L3/4 and L4/5, n = 32, Pfirrman score I/II/III, n = 25/3/4, respectively) and found a statistically significant negative correlation between |G*| and the Pfirrman score (r = -0.592, p = 0.0004), while no significant correlation was found for φ 9. Conversely, in a study of 47 subjects without current low back pain, Walter et al. showed that stiffness increased as IVD degeneration progressed 7.
Our study has limitations. First, only one IVD was investigated for each subject. Second, the Pfirrman score is a qualitative and subjective score and therefore prone to bias. However, it is the best noninvasive reference standard available, and the Pfirrman score was assessed by a board-certified radiologist specialized in musculoskeletal imaging. Third, as defined by the Pfirrman score, a clear distinction between nucleus and annulus is only possible in the healthy IVD (I) or in an early stage of degeneration (II), but not in progressing disease (≥III). Therefore, volumes of interest were drawn for the whole IVD and not separately for nucleus and annulus. Moreover, as the Pfirrman score V is defined as collapsed IVD space with hypointense or absent T2 signal, reduced anatomical landmarks complicated the outlining of volume of interests for this grade. Finally, there was an uneven distribution of Pfirrman scores which may have led to an overestimation of diagnostic performance.
Conclusion
This study shows an excellent diagnostic performance of multifrequency MRE with tomoelastography postprocessing for the assessment of lumbar spine IVD degeneration.Acknowledgements
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): SFB 1340/1 “Matrix in Vision” project number 372486779 (Bernd Hamm, Jürgen Braun, Ingolf Sack, Patrick Asbach), GRK 2260 BIOQIC (Ingolf Sack, Jürgen Braun), and RE 4161/2-1 (Rolf Reiter). Rolf Reiter is a participant of the BIH-Charité Digital Clinician Scientist Program funded by Charité – Universitätsmedizin Berlin, Berlin Institute of Health and the DFG.
References
1. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-304.
2. Patel AA, Spiker WR, Daubs M, Brodke D, Cannon-Albright LA. Evidence for an Inherited Predisposition to Lumbar Disc Disease. Journal of Bone and Joint Surgery. 2011;93(3):225-9.
3. Pfirrmann CWM, A; Zanetti, M; Hodler, J; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873-8.
4. James F Griffith Y-XJW, Gregory E Antonio, Kai Chow Choi, Alfred Yu, Anil T Ahuja, Ping Chung Leung. Modified Pfirrmann Grading System for Lumbar Intervertebral Disc Degeneration. Spine. 2007;32(24):E708-E12.
5. Co M, Dong H, Boulter DJ, Nguyen XV, Khan SN, Raterman B, et al. Magnetic Resonance Elastography of Intervertebral Discs: Spin-Echo Echo-Planar Imaging Sequence Validation. J Magn Reson Imaging. 2022.
6. Beauchemin PF, Bayly PV, Garbow JR, Schmidt JLS, Okamoto RJ, Cheriet F, et al. Frequency-dependent shear properties of annulus fibrosus and nucleus pulposus by magnetic resonance elastography. NMR Biomed. 2018;31(10):e3918.
7. Walter BA, Mageswaran P, Mo X, Boulter DJ, Mashaly H, Nguyen XV, et al. MR Elastography-derived Stiffness: A Biomarker for Intervertebral Disc Degeneration. Radiology. 2017;285(1):167-75.
8. Ben-Abraham EI, Chen J, Felmlee JP, Rossman P, Manduca A, An KN, et al. Feasibility of MR elastography of the intervertebral disc. Magn Reson Imaging. 2017;39:132-7.
9. Streitberger KJ, Diederichs G, Guo J, Fehlner A, Hamm B, Braun J, et al. In vivo multifrequency magnetic resonance elastography of the human intervertebral disk. Magn Reson Med. 2015;74(5):1380-7.
10. Cortes DH, Magland JF, Wright AC, Elliott DM. The shear modulus of the nucleus pulposus measured using magnetic resonance elastography: a potential biomarker for intervertebral disc degeneration. Magn Reson Med. 2014;72(1):211-9.
11. Streitberger K-J, Lilaj L, Schrank F, Braun J, Hoffmann K-T, Reiss-Zimmermann M, et al. How tissue fluidity influences brain tumor progression. Proceedings of the National Academy of Sciences. 2020;117(1):128-34.
12. Tzschatzsch H, Guo J, Dittmann F, Hirsch S, Barnhill E, Johrens K, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1-10.
Figures
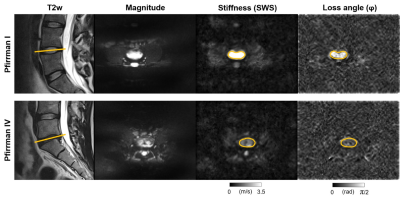
Figure 1. Upper row: Healthy 31-year-old man without disc degeneration (Pfirrman I) and shear wave speed (SWS) of 3.72 ± 0.90 m/s and loss angle (φ) of 1.02 ± 0.29 rad. Lower row: 30-year-old man with low back pain and advanced disc degeneration (Pfirrman IV) and SWS of 1.63 ± 0.33 m/s and φ of 0.67 ± 0.19 rad. Regions of interest are shown by orange circles and their location within the lumbar spine by orange lines.
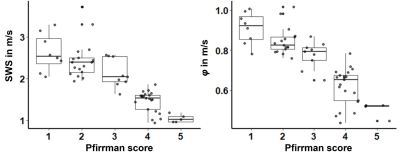
Figure 2. Box plots of shear wave speed (SWS) and loss angle (φ) according to Pfirrman scores.