2601
T1rho Dispersion in Proteoglycan-depleted Swine Spine Disc1Creighton University School of Medicine, Phoenix, AZ, United States, 2Translational Neuroscience, Barrow Neurological Institute, Phoenix, AZ, United States, 3Neurosurgery, Barrow Neurological Institute, Phoenix, AZ, United States, 4Sonntag Spine Center, Barrow Neurological Institute, Phoenix, AZ, United States, 5Radiology and Radiological Sciences, Vanderbilt Institute of Imaging Science, Nashville, TN, United States
Synopsis
Keywords: MSK, Degenerative, Intervertebral disc
T1rho imaging has been used to probe relatively slow macromolecular processes, making it a practical tool to gain information about water spin dynamics and interactions with endogenous macromolecules. We measured the dispersion of T1rho with different spin-lock fields in the intervertebral disc of swine spine specimens before and after the treatment to induce proteoglycan loss, a sign of early disc degeneration. Analysis revealed elevated T1rho values in all proteoglycan-depleted discs at each spin-lock field, and that post-treatment T1rho changes increase at a higher spin-lock field.INTRODUCTION
Degenerative disc disease (DDD) is a common cause of low back pain. Long before DDD can be seen using conventional diagnostic imaging, biochemical changes have already occurred1-3. Since proteoglycan loss in the nucleus pulposus (NP) is an early characteristic of disc degeneration causing a loss of water and height in the intervertebral disc (IVD), it is a prime biomarker for the early detection and treatment of DDD. T1rho imaging is sensitive to slow macromolecular interactions typically within the range of 0 - few KHz but varies with spin-lock field strength. Dispersion of T1rho provides novel information on dynamic processes within tissues, providing a more comprehensive analysis of the parameters of chemical exchange and/or intrinsic microstructure in DDD. Previous studies suggested that at higher static fields (3T and beyond) T1rho is strongly influenced by chemical exchange processes and diffusion within field inhomogeneities4,5. Recent studies suggested that T1rho at very low locking fields (≤ 200Hz) reflect diffusion of tissue water molecules within the field gradients caused by local magnetic field inhomogeneities. However, at higher locking fields, chemical exchange effects likely dominate6,7. Here, we investigated T1rho dispersion in swine disc specimens treated with Trypsin to induce proteoglycan depletion, to assess the sensitivity of using T1rho to detect low back pain.METHODS
Experiments were performed on a Philips 3T Ingenia scanner (Philips Healthcare, Best, The Netherlands). Fresh swine spine specimens were obtained from the neurosurgery research lab at the Barrow Neurological Institute. After removing debris, blood, muscle, and connective tissue, the specimen was soaked in PBS (phosphate buffered saline) solution for two days before MRI. After imaging, 0.1 mL Trypsin (Sigma-Aldrich, concentration: 25mg/mL) was injected into the nucleus pulposus by puncturing the anulus fibrosus with a 27G needle. Then the specimens were soaked in PBS at 4°C for two days prior to the post-treatment MRI. During imaging the disc was placed into a container containing a PBS solution to prevent dehydration and data was acquired with a 32-channel head coil (Philips Healthcare). A B0/B1 inhomogeneity self-compensated T1rho pre-pulse sequence8 was used to create T1rho contrast followed by Turbo Spin Echo (TSE) data acquisition. A single axial slice covering the disc area was chosen for imaging, with FOV: 245×245 mm2, pixel size: 0.5x0.5 mm2, slice thickness: 4 mm, TR/TE = 3000ms/10 ms, TSE factor = 15, NEX: 1. Five spin-lock times (TSLs) [1ms, 11ms, 21ms, 31ms, 41ms] were combined into a single scan for T1rho calculations, resulting in a scan time of 4min and 32sec. The T1rho experiment was repeated at different spin-lock frequencies (FSLs) [0Hz, 100Hz, 200Hz, 300Hz, 400Hz, 500Hz] to evaluate the T1rho dispersion in the disc pre- and post-treatment. After acquisition, a T1rho map at each spin-lock frequency was calculated by fitting the signal intensity vs TSL to a three-parameter mono-exponential model on a pixel-wise basis. Median values of the T1rho in the disc (nucleus pulposus) regions were used for comparisons.RESULTS
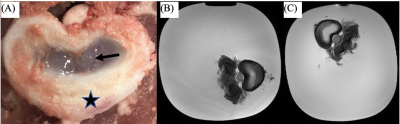
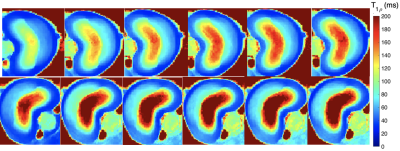
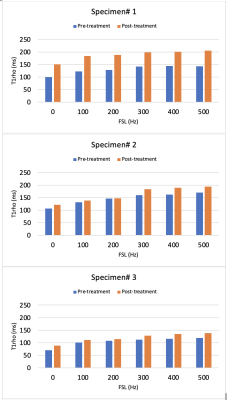
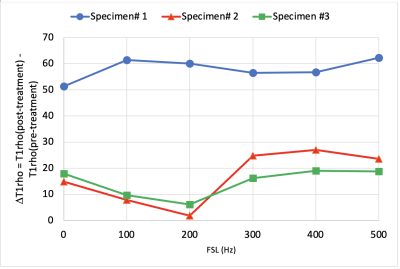
A picture of the swine disc sample, as well as T2W images pre- and post-treatment using Trypsin are shown in Fig. 1. Comparisons of T1rho maps at each spin-lock fields (i.e., T1rho dispersion) pre- and post-treatment showing increased T1rho with spin-lock frequency (Fig. 2), which was also plotted in Fig.3. There was a consistent T1rho increase after proteoglycan depletion at each spin-lock frequency. Finally, the T1rho change (between pre- and post-treatment) at different spin-lock frequencies was shown in Fig.4.DISCUSSION
T1rho dispersion imaging provides a more complete characterization of tissue composition and physicochemical changes associated with DDD pathology. Although we found that T1rho increases after Trypsin induced proteoglycan-depletion, similar to that seen in cartilage in osteoarthritis9,10, the results differed from a report that T1rho decrease after Chondroïtinase ABC induced proteoglycan loss2. We speculate that the increase of T1rho in disc may be attributed to the breakdown of the proteoglycan by Trypsin, which leads to an overall shorter correlation time (longer T1rho time). In addition, T1rho at very low locking fields (≤200Hz) may reflect diffusion of tissue water molecules, and at higher locking fields, chemical exchange effects dominate6. Based on our preliminary data, we found an increase in the difference for T1rho between pre- and post-treatment for locking fields greater than 200Hz. Our long term goal is to improve the sensitivity of T1rho dispersion (i.e., the T1rho difference between two spin-locking fields) to the proteoglycan depletion to provide a more accurate diagnosis and management of low back pain.CONCLUSIONS
The study showed that T1rho values increase after the proteoglycan depletion treatment at all spin-lock fields examined. Greater spin-lock frequencies may be more beneficial to detect changes associated with proteoglycan loss. More research is needed to utilize T1rho dispersion to characterize tissue composition and physicochemical changes associated with DDD.Acknowledgements
Barrow Neurological Foundation (Award Number: 455003033568).References
1. de Oliveira CP, Rodrigues LM, Fregni MV, et al. Extracellular matrix remodeling in experimental intervertebral disc degeneration. Acta Ortop Bras. 2013;21(3):144-149.
2. Paul CPL, Smit TH, de Graaf M, et al. Quantitative MRI in early intervertebral disc degeneration: T1rho correlates better than T2 and ADC with biomechanics, histology and matrix content. PLoS One. 2018;13(1): e0191442.
3. Tanaka N, An HS, Lim TH, et al. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1(1):47-56.
4. Cobb J, Xie J, Li K, et al. Exchange-mediated contrast agents for spin-lock imaging. MRM. 2012; 67(5):1427-1433.
5. Cobb J, Li K, Xie J, et al. Exchange-mediated contrast in CEST and spin-lock imaging. MRI. 2014; 32(1):28-40. 2015;7(12):1269-1281.
6. Adelnia F, Zu Z, Spear JT, et al. Tissue characterization using R1rho dispersion imaging at low locking fields. Magn Reson Imaging 2021;84:1-11.
7. Zu Z, Janve V, Gore JC. Spin-lock imaging of intrinsic susceptibility gradients in tumors. Magn Reson Med. 2020;83:1587-1595.
8. Witschey W, Borthakur A, Elliott M, et al. Artifacts in T1ρ-Weighted Imaging: Compensation for B1 and B0 Field Imperfections. JMR. 2007;186(1):75-85.
9. Borthakur A, Mellon E, Niyogi S, et al. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781-821.
10. Goto H, Iwama Y, Fujii M, et al. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol. 2012;81(7):e796-803.
Figures