2593
Feasibility of high-resolution multi-shot DW-EPI for DTI of peripheral nerves in the knee1Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Neurography, Diffusion Tensor Imaging, Multi-shot DW-EPI, repeatability, feasibility
DTI of peripheral nerves for detecting nerve damage has been very challenging due to limited resolution and imaging artifacts of the conventional single-shot diffusion-weighted echoplanar imaging (SS-DW-EPI). Here, we have evaluated the feasibility of improved DTI with multi-shot DW-EPI (MUSE) by comparing mean diffusivity (MD) and fractional anisotropy (FA) estimates of tibial and peroneal nerves with those from SS-DW-EPI in ten healthy subjects. MUSE showed lower within-subject coefficient of variation in diffusion estimates, indicating improved repeatability, while correlation analysis showed noticeable differences between FA estimates. Our results show the feasibility of MUSE for in vivo evaluation of peripheral nerves.Introduction
Fat-suppressed images from T2-weighted fast spin-echo have been increasingly used in clinics for locating abnormal peripheral nerves. Unfortunately, T2 contrast only indicates that there might be some form of nerve abnormality and cannot determine a specific pathology. Quantitative diffusion estimates from diffusion tensor imaging (DTI) have demonstrated a promising correlation with demyelination and axonal loss in previous preclinical and clinical studies1-4. However, the conventional acquisition method of DTI (single-shot diffusion-weighted EPI, SS-DW-EPI) has limited resolution as well as artifacts that prevent reliable delineation of small peripheral nerves in in vivo imaging cases.Multi-shot diffusion-weighted EPI using multiplexed sensitivity encoding (MUSE)5 has enabled significantly higher resolution DTI with reduced distortion artifacts than SS-DW-EPI. In this study, we investigated the feasibility of DTI with MUSE for peripheral nerves. We measured the repeatability of its diffusion estimates (MD and FA) from the tibial and peroneal nerves at the knee of ten healthy subjects and compared with those from SS-DW-EPI. We also measured the correlation of the diffusion estimates between these two sequences.
Methods
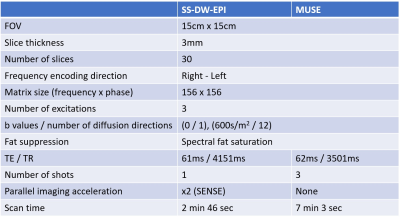
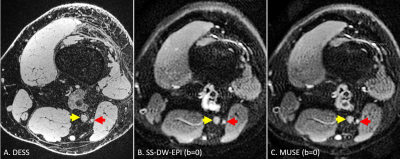
Ten healthy controls (three males, seven females, mean age: 34.3 years, age range: 23 years – 53 years) participated in our study. We scanned their knee in a 3T GE Premier MRI scanner using a 16-channel flex coil. A high-resolution (0.5mm x 0.6mm x 0.5mm) sagittal 3D double-echo in a steady state (DESS) sequence was run before our main DTI sequences to obtain an anatomical reference. The main DTI sequences included an axial SS-DW-EPI sequence and an axial three-shot MUSE sequence, with each sequence repeated twice. Both SS-DW-EPI and MUSE shared the same FOV (15cm x 15cm), in-plane resolution (1mm x 1mm), slice thickness (3.0mm), and diffusion encoding parameters (b = 600 s/mm2). The detailed sequence parameters are described in Figure 1. The most superior slice of diffusion sequences was placed at a point where the sciatic nerve bifurcates into the tibial and peroneal nerves to help identify each nerve.For each subject, a single slice distal to the bifurcation point, as shown in Figure 2, was chosen to place an ROI for the tibial nerve (yellow arrow) and the peroneal nerve (red arrow) on the MD and FA maps from DTI sequences. Each ROI’s average MD and FA values were measured and used for the following analyses. For correlation analysis, we have computed the Pearson correlation coefficient of these diffusion estimates between SS-DW-EPI and MUSE. For repeatability analysis, we have calculated the within-subject coefficient of variation (wsCOV) for MD and FA values and formed Bland-Altman plots.
Results
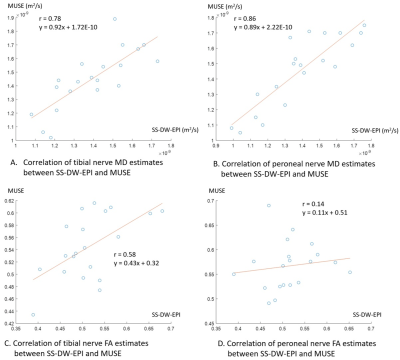
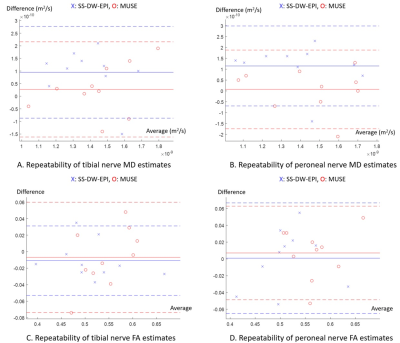
Figure 3 illustrates the correlation of MD and FA estimates of the tibial and peroneal nerves between SS-DW-EPI and MUSE. While MD estimates had decently high Pearson correlation coefficients (r = 0.78 for tibial nerve, r = 0.86 for peroneal nerve), FA estimates had low Pearson correlation coefficients (r=0.58 for tibial nerve, r = 0.14 for the peroneal nerve). Figure 4 shows Bland-Altman plots comparing MD and FA estimates between repeated SS-DW-EPI and MUSE scans. MD estimates from SS-DW-EPI had a larger bias in the mean differences than MUSE, while standard deviations were similar in both tibial and peroneal nerves. FA estimates showed similar biases in the mean differences for both SS-DW-EPI and MUSE, but the standard deviation was smaller for SS-DW-EPI on the tibial nerve. Figure 5 summarizes wsCOV values, demonstrating that MUSE showed consistently lower wsCOV values, thus higher repeatability, except for the tibial nerve FA case.Discussion
In this work, we have compared the MD and FA estimates of the tibial and peroneal nerves at the knee between SS-DW-EPI with 2x parallel imaging acceleration and 3-shot MUSE sequences. The correlation of diffusion estimates between the two sequences was high for MD but low for FA, with MUSE generating higher estimates for MD and FA than SS-DW-EPI in many cases. The higher SNR of MUSE will likely reduce the underestimation of a high diffusion coefficient due to the false signal elevation at the noise floor. Thus MUSE may recover high diffusion coefficients better than SS-DW-EPI, which can produce a higher difference in FA estimates than MD estimates because FA captures the differences of diffusion coefficients between major diffusion directions while MD averages them. Bland-Altman analyses of FA and MD estimates showed that both sequences achieved reasonable repeatability, whereas MUSE achieved smaller bias in MD estimates. Smaller wsCOV values of MUSE than those of SS-DW-EPI in all diffusion estimates except for tibial nerve FA also demonstrate the potential for improved repeatability with MUSE, supporting its feasibility for in vivo peripheral nerve imaging studies.Conclusion
In this work, we investigated the feasibility of DTI with MUSE for the tibial and peroneal nerves by comparison with SS-DW-EPI in ten healthy subjects. MUSE achieved improved repeatability in most MD and FA estimates with a tendency to generate higher MD and FA estimates than SS-DW-EPI, likely due to higher SNR. Our results show the promising potential of MUSE for in vivo evaluation of peripheral nerves.Acknowledgements
This work was supported by GE Healthcare and NIH R01-AR077604.References
1. Gallagher TA, Simon NG, & Kliot M (2015) Diffusion tensor imaging to visualize axons in the setting of nerve injury and recovery. Neurosurg Focus 39(3):E10.
2. Kakuda T, et al. (2011) Diffusion tensor imaging of peripheral nerve in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a feasibility study. Neuroradiology 53(12):955-960.
3. Morisaki S, et al. (2011) In vivo assessment of peripheral nerve regeneration by diffusion tensor imaging. J Magn Reson Imaging 33(3):535-542.
4. Heckel A, et al. (2015) Peripheral Nerve Diffusion Tensor Imaging: Assessment of Axon and Myelin Sheath Integrity. PLoS One 10(6):e0130833.
5. Chen NK, Guidon A, Chang HC, & Song AW (2013) A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 72:41-47.
Figures