2587
Resting state neurovascular coupling patterns estimated using advanced multiband multi-echo BOLD/ASL imaging1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Arterial spin labelling, Data Analysis, Coupling, Simultaneous BOLD/ASL
Impaired neurovascular coupling (NVC) plays a critical role in many neurovascular pathological processes. Several resting state fMRI methods have been used to estimate NVC including BOLD/CBF coupling and, to a lesser extent, ALFF and fALFF. In this study, resting state BOLD/CBF coupling was evaluated using advanced multiband multi-echo BOLD/ASL sequence and correlated with ALFF and fALFF in healthy volunteers. Significant correlation between BOLD/CBF coupling and ALFF and fALFF was seen in major brain network hubs. These results indicate MBME BOLD/ASL may provide similar, but complimentary measures of NVC compared to traditional RS metrics.
Introduction
Neurovascular coupling (NVC), where acute localized blood flow increases following neural activity, is the basis for the BOLD response in fMRI1. Impaired NVC plays a critical role in a number of neurovascular pathological processes. Recent technical developments have combined a multiband acquisition with four total echoes with a pseudocontinuous arterial spin labelling (pCASL) approach to simultaneously collect BOLD and cerebral blood flow (CBF) data2. This sequence can be used to measure BOLD/CBF coupling as an estimate of NVC. In addition, traditional resting state metrics such as ALFF and fALFF can be measured with the same acquisition. These metrics have been suggested as potential surrogates for NVC. The relationship between BOLD/CBF coupling and these RSFC metrics is largely unknown. In this study, resting state BOLD/CBF coupling was evaluated and correlated with ALFF and fALFF in a group of healthy volunteers.Methods
Twenty-nine healthy volunteer subjects (Mean Age=28.0 Range 20 – 46, 9 Male, 20 Female) participated in this study. Imaging was performed on a 3T scanner. Each subject underwent a multiband multi-echo BOLD/ASL resting state fMRI acquisition2,3 with the following parameters: TR/TE=3500/11,30,49,67ms, FOV=24cm, matrix size=80x80 with slice thickness=3mm (3x3x3mm voxel size), 11 slices with multiband factor=4 (44 total slices), FA=90°, and partial Fourier factor=0.85. The sequence also incorporated an unbalanced pCASL labeling scheme with labeling time=1.5s and PLD=1.0s. Resting state scans used a single shot EPI readout with in-plane acceleration (R)=2 and lasted six minutes resulting in 103 volumes.Data was analyzed using a combination of AFNI4,5, and FSL6. First, the anatomical MPRAGE image was coregistered to Montreal Neurological Institute (MNI) space. Then, the first-echo functional data was volume-registered to the first volume. Subsequent echoes were registered using the transformation matrices from the first echo. For the BOLD data, the four echoes were combined using the -weighted approach7 and denoised using multi-echo independent component analysis (ME-ICA)8-10 by regressing non-BOLD independent components out of the combined ME data. The denoised MBME dataset was then registered to the MPRAGE image and then to MNI space using the anatomical transformations computed above. Finally, the denoised data was bandpass filtered with 0.01 < f < 0.071Hz corresponding to 1/(4*TR). A perfusion-weighted (PW) timeseries was generated by highpass filtering the first echo at f > 0.071Hz followed by demodulation11.
The BOLD/CBF coupling was assessed by correlating the signals from the BOLD and PW datasets on a voxelwise basis using Pearson correlation with 3dTcorrelate in AFNI. The BOLD time series was time-shifted from -2TR to +2TR (-7.0 – 7.0s) with steps of 1 TR. The voxelwise maximum correlation within this range was defined as rmax. ALFF and fALFF were computed using 3dRSFC in AFNI for the BOLD data. To examine the relationship between BOLD/CBF coupling and the traditional RS metrics, rmax was correlated with ALFF and fALFF on a voxelwise basis across subjects using 3dTcorrelate in AFNI with Pearson correlation. The resulting correlation maps were thresholded at p < 0.05 with cluster size correction for multiple comparisons.
Results
Individual subject and group-averaged BOLD/CBF coupling results are shown in Figure 1. Heightened coupling was widespread, with coupling highest in the parietal, frontal and visual regions. Group averaged ALFF and fALFF metrics are shown in Figure 2. The results of the correlation between BOLD/CBF coupling (rmax) and traditional resting state metrics (ALFF and fALFF) are shown in Figure 3. Significant correlation between BOLD/CBF coupling and ALFF was seen mainly in the visual cortex and motor cortex. Similar results were seen for the correlation between BOLD/CBF coupling and fALFF, however significant correlation was more widespread including more parietal and frontal areas.Discussion/Conclusions
The group average for BOLD/CBF coupling showed stronger coupling in similar areas as previous studies including default mode network (DMN), visual cortex and frontal areas12,13. In order to tease apart the relationship between BOLD/CBF coupling and NVC, coupling was correlated with traditional RS metrics including ALFF and fALFF. ALFF has been shown to correlate with cerebrovascular reactivity (CVR) a measure of vascular health that has been used to estimate NVC14,15. The CBF/ALFF ratio has also been shown to be altered in disease16 as has the CBF/fALFF ratio17. Finally, Tak et al found coupling correlated with RSFC strength in the DMN18. Our findings showed BOLD/CBF coupling was significantly correlated with traditional RS metrics including ALFF, and fALFF in major network hubs indicating coupling may provide similar, but complimentary measures of NVC compared to traditional RS metrics. The multiband multi-echo BOLD/ASL approach could provide a useful tool for estimating NVC in translational neuroscience research, however studies in pathological conditions are needed.Acknowledgements
No acknowledgement found.References
1. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America 1990;87(24):9868-9872.
2. Cohen AD, Nencka AS, Lebel RM, Wang Y. Multiband multi-echo imaging of simultaneous oxygenation and flow timeseries for resting state connectivity. PloS one 2017;12(3):e0169253.
3. Cohen AD, Nencka AS, Wang Y. Multiband multi-echo simultaneous ASL/BOLD for task-induced functional MRI. PloS one 2018;13(2):e0190427.
4. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29(3):162-173.
5. Cox RW. AFNI: what a long strange trip it's been. NeuroImage 2012;62(2):743-747.
6. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage 2012;62(2):782-790.
7. Posse S, Wiese S, Gembris D, Mathiak K, Kessler C, Grosse-Ruyken ML, Elghahwagi B, Richards T, Dager SR, Kiselev VG. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 1999;42(1):87-97.
8. Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences of the United States of America 2013;110(40):16187-16192.
9. Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage 2012;60(3):1759-1770.
10. DuPre E, Salo T, Markello R, Kundu P, Whitaker K, Handwerker D. ME-ICA/tedana: 0.0.6: https://doi.org/10.5281/zenodo.2558498. 2019.
11. Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, Duyn JH, Talagala SL. Mapping resting-state functional connectivity using perfusion MRI. NeuroImage 2008;40(4):1595-1605.
12. Tak S, Wang DJJ, Polimeni JR, Yan L, Chen JJ. Dynamic and static contributions of the cerebrovasculature to the resting-state BOLD signal. NeuroImage 2014;84:672-680.
13. Chiacchiaretta P, Cerritelli F, Bubbico G, Perrucci MG, Ferretti A. Reduced Dynamic Coupling Between Spontaneous BOLD-CBF Fluctuations in Older Adults: A Dual-Echo pCASL Study. Front Aging Neurosci 2018;10:115.
14. Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Frontiers in human neuroscience 2013;7:118.
15. Kazan SM, Mohammadi S, Callaghan MF, Flandin G, Huber L, Leech R, Kennerley A, Windischberger C, Weiskopf N. Vascular autorescaling of fMRI (VasA fMRI) improves sensitivity of population studies: A pilot study. NeuroImage 2016;124(Pt A):794-805.
16. Hu B, Yan L-F, Sun Q, Yu Y, Zhang J, Dai Y-J, Yang Y, Hu Y-C, Nan H-Y, Zhang X. Disturbed neurovascular coupling in type 2 diabetes mellitus patients: evidence from a comprehensive fMRI analysis. NeuroImage: Clinical 2019;22:101802.
17. Li P, Mu J, Ma X, Ding D, Ma S, Zhang H, Liu J, Zhang M. Neurovascular coupling dysfunction in end-stage renal disease patients related to cognitive impairment. Journal of Cerebral Blood Flow & Metabolism 2021;41(10):2593-2606.
18. Tak S, Polimeni JR, Wang DJ, Yan L, Chen JJ. Associations of resting-state fMRI functional connectivity with flow-BOLD coupling and regional vasculature. Brain connectivity 2015;5(3):137-146.
Figures
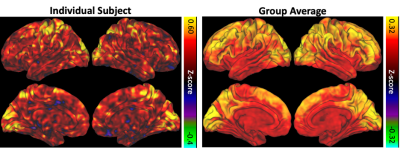
Figure 1. BOLD/CBF coupling for a representative individual subject (left) and averaged across the entire group (right).
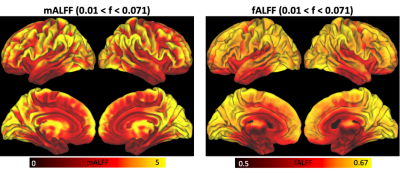
Figure 2. ALFF (left) and fALFF (right) averaged across the group.
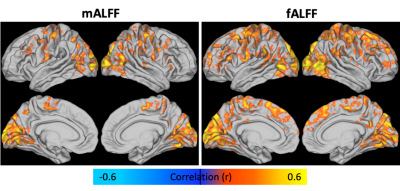
Figure 3. Spatial correlation of BOLD/CBF coupling (rmax) with mALFF (left) and fALFF (right). Similar regions were observed for both metrics, while fALFF correlations were more widespread.