2580
Impact of age and sex on regional white matter hemodynamics across the adult lifespan1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Neuroimaging Research for Veterans Center, VA Boston Healthcare System, Boston, MA, United States
Synopsis
Keywords: Arterial spin labelling, Aging
White matter perfusion has been difficult to measure with arterial spin labeling (ASL) MRI due to the elongated arterial transit times of white matter. Here, we identified changes in white matter cortical hemodynamics associated with age and between sex for the first time using multi-delay pseudo-continuous ASL with tract-based analyses in a large typically aging cohort. We found reductions in white matter CBF and increases in white matter ATT with advancing age, as well as widespread sex differences across tracts. This work serves as the first step towards understanding the relationship between white matter physiology and age-related structural decline.Introduction
White matter has lower vascular density and longer blood transit time in comparison to gray matter, making blood flow difficult to detect within tracts using arterial spin labeling (ASL) MRI1. Recently, multi-delay ASL with high spatial resolution was introduced to the Lifespan Human Connectome Project in Aging (HCP-A), enabling better identification of hemodynamic signals in white matter2. Previous work has investigated global white matter in the HCP-A cohort, with cerebral blood flow (CBF) demonstrating non-linear associations with age and arterial transit time (ATT) increasing with age3. However, white matter vascular density varies across tracts and the location and burden of white matter lesions can greatly affect cognitive ability and the progression of neurodegenerative disorders4. Here, we investigated associations between white matter perfusion and age in HCP-A using a tract-based approach.Methods
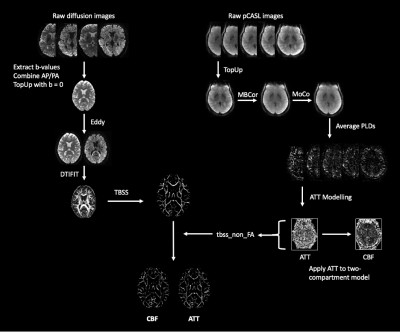
Participants. Publicly available HCP-A data in which diffusion and perfusion imaging, as well as information on age and sex, were available amounted to 678 participants (377 female; 296 male). The demographics of the HCP-A cohort have been described in depth previously5. Imaging data were collected at 3T (Siemens Prisma) across four sites and participants provided informed written consent.Data acquisition and processing. Acquisition details for all scans have been detailed in the literature2. However, parameters and processing of interest for diffusion and perfusion imaging are detailed below and summarized in Figure 1.
Perfusion: 2D pseudo-continuous ASL with multi-band 2D gradient echo echo-planar imaging (GRE-EPI) readout, labeling duration=1500 ms, five post-labeling delays (PLD) of length=200 ms (control/label pairs=6), 700 ms (pairs=6), 1200 ms (pairs=6), 1700 ms (pairs=10), and 2200 ms (pairs=15), TR=3580 ms, TE=19 ms, partial Fourier=6/8, simultaneous multi-slice (SMS) acceleration factor=6, total slices=60, spatial resolution=2.5×2.5×2.5 mm3, total acquisition time=5.5 min. Equilibrium magnetization (M0) images were acquired at the end of the scan, as well as two spin echo EPI scans with opposite phase encoding directions for distortion correction, with TR=8000 ms, TE=40 ms, spatial resolution=2.5×2.5×2.5 mm3. Images were corrected for distortions using 'TopUp'6, corrected for motion using AFNI '3dvolreg'7 and corrected for multi-band artifacts using slice interpolation. Partial volume correction was also applied8. CBF and ATT quantification was performed based on an approach described in previous work3.
Diffusion: Diffusion-weighted data was acquired using a pulsed-gradient spin-echo EPI sequence with opposing phase-encoding directions: b-values=0,1.5 ms/μm2, 99 and 98 diffusion encoding directions, 1.5 mm isotropic resolution, TR=3230 ms, TE=89.2 ms, SMS factor=4, no in-plane acceleration. 'TopUp' was used for distortion correction, with brain mask generation in AFNI '3dSkullStrip'7 and eddy and motion correction was performed using FSL 'eddy'9. FSL 'DTIFIT' was then used for extracting fractional anisotropy (FA) maps, and tract-based spatial statistics was used to extract the white matter skeleton for projecting the CBF and ATT maps onto the white matter structure10.
Data analysis. Results were computed using FSL 'randomise', with a general linear model for sex and age effects11. Multiple comparisons correction was performed using threshold-free cluster enhancement (TFCE) family-wise error (FWE) correction (pFWE<0.05) and a conservative voxel-wise correction (pFWE<0.05) to visualize significant effects. The JHU white matter and tract atlases were used to localize changes observed12.
Results
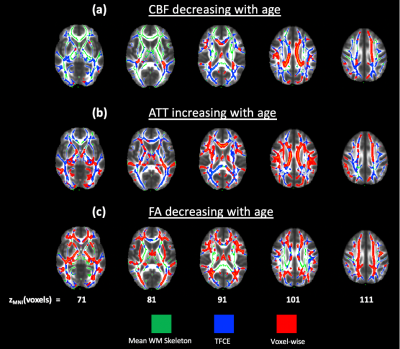
Impact of age on FA. Figure 2 demonstrates widespread significant (pFWE<0.05) FA decreases with age, with the most apparent regions after conservative thresholding including the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, corpus callosum, external capsule and fornix. However, significant increases in FA associated with age were also seen in the retrolenticular part of the internal capsule, superior cerebellar peduncle and body of the corpus callosum.Impact of age on white matter perfusion. Figure 3 shows decreases in CBF associated with age in the body and splenium of the corpus callosum, corona radiata and internal capsule, whereas more widespread ATT increases associated with age were seen, particularly in the cingulum.
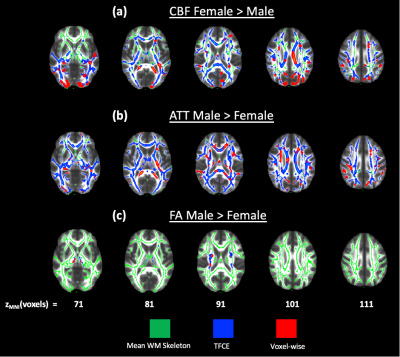
Impact of sex on white matter perfusion. Figure 4 presents tracts with sex differences in CBF, ATT and FA. Women demonstrate elevated CBF in the fornix and uncinate fasciculus, whereas men demonstrate elevated ATT in the corona radiata. Few differences in FA were found between sex, with men demonstrating higher FA in the corticospinal tract.
Discussion
Decreases in FA with age have been observed previously and are likely related to age-related myelin breakdown13. Though decreases in CBF and increases in ATT associated with age have been observed globally in white matter3,14, it is evident that these effects vary by tract. More tracts demonstrate ATT changes with age compared to CBF, which could support that ATT may be more sensitive to hemodynamic compromise3. These results were detectable due to the sensitivity of multi-delay pCASL to the longer transit times of white matter. Sex differences in ATT and CBF also agree with the existing literature on global white matter3,15.In conclusion, this is the first study within HCP-A to identify changes in white matter perfusion with age and between sex using tractography. This work could help characterize the aging process and reveal potential underlying physiological mechanisms behind white matter degeneration. Future directions could include investigating the influence of lifestyle factors, cognition and medical history on white matter perfusion, to better identify the role of physiological changes within tracts.
Acknowledgements
This work was supported by the National Institutes of Health/National Institute on Aging (R21AG072068, U01AG052564, and U01AG052564-S1) and the American Heart Association (19CDA34790002). This research was made possible in part by the computational hardware generously provided by the Massachusetts Life Sciences Center (https://www.masslifesciences.com/).References
1. Hase, Y., Ding, R., Harrison, G., Hawthorne, E., King, A., Gettings, S., Platten, C., Stevenson, W., Craggs, L.J. and Kalaria, R.N., 2019. White matter capillaries in vascular and neurodegenerative dementias. Acta neuropathologica communications, 7(1), pp.1-12.
2. Harms, M.P., Somerville, L.H., Ances, B.M., Andersson, J., Barch, D.M., Bastiani, M., Bookheimer, S.Y., Brown, T.B., Buckner, R.L., Burgess, G.C. and Coalson, T.S., 2018. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage, 183, pp.972-984.
3. Juttukonda, M.R., Li, B., Almaktoum, R., Stephens, K.A., Yochim, K.M., Yacoub, E., Buckner, R.L. and Salat, D.H., 2021. Characterizing cerebral hemodynamics across the adult lifespan with arterial spin labeling MRI data from the Human Connectome Project-Aging. Neuroimage, 230, p.117807.
4. Filley, C.M. and Fields, R.D., 2016. White matter and cognition: making the connection. Journal of neurophysiology, 116(5), pp.2093-2104.
5. Bookheimer, S.Y., Salat, D.H., Terpstra, M., Ances, B.M., Barch, D.M., Buckner, R.L., Burgess, G.C., Curtiss, S.W., Diaz-Santos, M., Elam, J.S. and Fischl, B., 2019. The lifespan human connectome project in aging: an overview. Neuroimage, 185, pp.335-348.
6. Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E., Johansen-Berg, H., Bannister, P.R., De Luca, M., Drobnjak, I., Flitney, D.E. and Niazy, R.K., 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, pp.S208-S219.
7. Cox, R.W., 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research, 29(3), pp.162-173.
8. Greve, D.N., Salat, D.H., Bowen, S.L., Izquierdo-Garcia, D., Schultz, A.P., Catana, C., Becker, J.A., Svarer, C., Knudsen, G.M., Sperling, R.A. and Johnson, K.A., 2016. Different partial volume correction methods lead to different conclusions: an 18F-FDG-PET study of aging. Neuroimage, 132, pp.334-343.
9. Andersson, J.L. and Sotiropoulos, S.N., 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, pp.1063-1078.
10. Smith, S.M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., Watkins, K.E., Ciccarelli, O., Cader, M.Z., Matthews, P.M. and Behrens, T.E., 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), pp.1487-1505.
11. Winkler, A.M., Ridgway, G.R., Webster, M.A., Smith, S.M. and Nichols, T.E., 2014. Permutation inference for the general linear model. Neuroimage, 92, pp.381-397.
12. Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D.S., Calabresi, P.A., Pekar, J.J., van Zijl, P.C. and Mori, S., 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage, 39(1), pp.336-347.
13. Bartzokis G., 2011. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 32: 1341–1371.
14. Wright, S. N., Kochunov, P., Chiappelli, J., McMahon, R. P., Muellerklein, F., Wijtenburg, S. A., White, M. G., Rowland, L. M., & Hong, L. E., 2014. Accelerated white matter aging in schizophrenia: role of white matter blood perfusion. Neurobiology of Aging, 35(10), 2411–2418.
15. Alisch, J.S., Khattar, N., Kim, R.W., Cortina, L.E., Rejimon, A.C., Qian, W., Ferrucci, L., Resnick, S.M., Spencer, R.G. and Bouhrara, M., 2021. Sex and age-related differences in cerebral blood flow investigated using pseudo-continuous arterial spin labeling magnetic resonance imaging. Aging (Albany NY), 13(4), p.4911.
Figures