2559
Breast cancer diagnosis and prognosis by using a high b-value continuous-time random-walk model1Department of Radiology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China, 2Shenzhen United Imaging Research Institute of Innovative Medical Equipment, Shenzhen, China, 3Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronics Science, East China Normal University, Shanghai, China, 4Department of Anesthesiology, The Fourth Hospital of Shijiazhuang, Shijiazhuang, China
Synopsis
Keywords: Breast, Diffusion/other diffusion imaging techniques
Breast cancer is a common cancer that severely threatens the health of women worldwide. The advanced diffusion model using high b-values enables a more comprehensive description of the tumor tissue by cellularity and heterogeneity. In current study, a continuous-time random-walk (CTRW) model was applied to identify malignancy of breast lesions and the association between model-derived parameters and immunohistochemical indices was evaluated. All model-derived parameters could identify tumor malignancy, combined parameter could further discriminate ER+/ER- and PR+/PR- patients, while temporal heterogeneity parameter was significantly correlated with PR expression. The CTRW model has demonstrated potential value in breast cancer diagnosis and prognosis.Introduction
Breast cancer is the most common cancer in the female population worldwide1. Previous studies have demonstrated that hormone receptor expression of patients with malignant breast lesion not only guides clinical treatment options, but also correlates with prognosis2. In recent years, other than conventional mono-exponential diffusion model, advanced diffusion models that involve high b-values and are capable of describing the non-Gaussian diffusion process of water molecules have been increasingly applied to cancer researches. Lately, a new continuous-time random-walk (CTRW) model has been proven to be a useful diffusion model that could be used to assess both cellularity and heterogeneity of tissue3,4. However, the application of the CTRW model in breast cancer diagnosis and prognosis has not been evaluated. Here, we investigated whether the CTRW model-derived parameters could identify malignancy of breast tumor, and evaluated the association between diffusion parameters and prognosis-related immunohistochemical indices.Methods
Patients:A total of 85 patients (mean age ± standard deviation: 48.42 ± 11.63 years) were recruited with approval from the institutional review board and written informed consent, 51 of whom had malignant breast lesions. Image acquisition: The MRI examinations were performed on a 3.0 T scanner (uMR 780, United Imaging Healthcare, Shanghai, China). The protocols include: 1) Axial T1-weighted fast spin-echo sequence (T1WI); 2) Axial T2-weighted fast spin-echo sequence (T2WI); 3) Multiple b-value echo planar imaging (b = 0, 50, 100, 200, 400, 600, 800, 1000, 2000, 3000 $$$s/mm^{2}$$$) sequence.
Diffusion Model Fitting:
Diffusion parameters were fitted using following equations:
1) mono-exponential model,
$$\frac{S}{S_0}=e^{-b*ADC}$$
where and are the signal intensity with b-value of 0 and 800 $$$s/mm^{2}$$$, respectively; represents the apparent diffusion coefficient.
2) CTRW model,
$$\frac{S}{S_0}=E_{\alpha}\left(-\left(bD_m\right)^{\beta}\right)$$
where $$$S_0$$$ is the signal intensity when b-value = 0 $$$s/mm^{2}$$$, $$$S$$$ is the signal intensity with other b-values, $$$E_{\alpha}$$$ is Mittag-Leffler function, $$$D_m$$$ is anomalous diffusion coefficient, $$$\alpha$$$ and $$$\beta$$$ are temporal and spatial heterogeneity parameters, respectively.
Regions of interest (ROIs) that only contain solid parts of tumor were delineated manually based on the complementary information of DWI and T2WI images by two senior radiologists.
Statistical analysis:
Mann-Whitney U-test was used for the comparison of diffusion parameters. The combined parameters were generated with all parameters derived from CTRW model alone or together with mono-exponential model-derived . Receiver operating characteristic (ROC) curves were plotted in identifying ER+/ER- and PR+/PR- patients, further spearman correlation analysis was performed to investigate the association between diffusion parameters and immunohistochemical indices expression.
Results
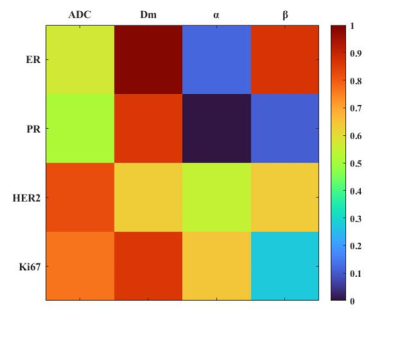
Figure 1 illustrated a set of representative images including T2 image, diffusion image with b = 800 $$$s/mm^{2}$$$ and parametric maps of $$$ADC$$$,$$$D_m$$$, $$$\alpha$$$ and $$$\beta$$$ for a malignant case. All diffusion parameters were significantly lower in malignant lesions compared to the benign lesion group (P < 0.01 for $$$ADC$$$,$$$D_m$$$, $$$\alpha$$$ and $$$\beta$$$), as shown in Figure 2. The combination of $$$D_m$$$, $$$\alpha$$$ and $$$\beta$$$ had the largest AUC (AUC = 0.969, P < 0.01) in the discrimination of malignancy of breast lesions (Table 1). The combination of $$$ADC$$$ and all CTRW model-derived parameters could effectively discriminate ER+/ER- and PR+/PR- patients (AUC = 0.750, 0.792; P < 0.01, P < 0.01), as displayed in Figure 3. Figure 4 demonstrated a significant positive correlation between $$$\alpha$$$ and the PR expression (P < 0.01).Discussion
In this study, the CTRW diffusion model was applied in the diagnosis and prognosis of breast cancer. The parameters derived from CTRW model were all able to effectively identify the benign and malignant breast lesions. Although $$$D_m$$$ had similar performance with conventional $$$ADC$$$, the combination of $$$D_m$$$, $$$\alpha$$$ and $$$\beta$$$ had the largest AUC value, slightly higher than single parameters. Importantly, the combination of $$$ADC$$$ and all CTRW model-derived parameters could discriminate ER+/ER- and PR+/PR- patients. Meanwhile, the temporal heterogeneity index $$$\alpha$$$ performed a significant positive correlation with PR expression in PR+ patients.Conclusion
Our results indicated that CTRW model-derived parameters can distinguish benign and malignant breast lesions. The validity of the combined diffusion parameter for the identification of hormone receptor (ER/PR) positive patients and the association between and PR expression suggests that the CTRW model has promising applications in prognosis and treatment decision of breast cancer.Acknowledgements
No acknowledgement found.References
1. Watkins EJ. Overview of breast cancer. JAAPA. Oct 2019;32(10):13-17. doi:10.1097/01.JAA.0000580524.95733.3d
2. Clark BZ, Dabbs DJ, Cooper KL, Bhargava R. Impact of progesterone receptor semiquantitative immunohistochemical result on Oncotype DX recurrence score: a quality assurance study of 1074 cases. Appl Immunohistochem Mol Morphol. Jul 2013;21(4):287-91. doi:10.1097/PAI.0b013e31826f80c9
3. Karaman MM, Sui Y, Wang H, Magin RL, Li Y, Zhou XJ. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med. Oct 2016;76(4)(CTRW):1149-57. doi:10.1002/mrm.26012
4. Zhong Z, Merkitch D, Karaman MM, et al. High-Spatial-Resolution Diffusion MRI in Parkinson Disease: Lateral Asymmetry of the Substantia Nigra. Radiology. Apr 2019;291(1) 149-157. doi:10.1148/radiol.2019181042
Figures
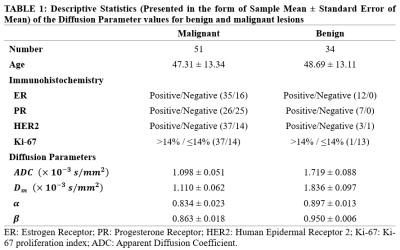
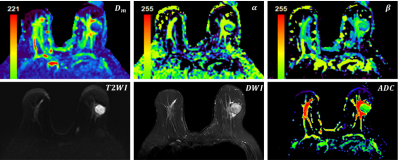
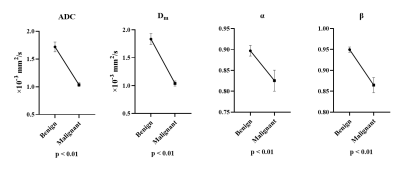
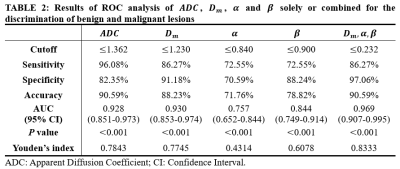
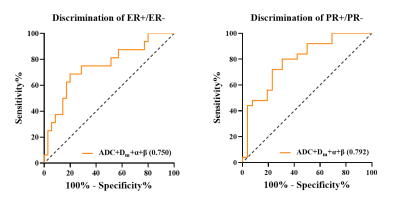
Figure 3: ROC curves of combined parameter in distinguishing between ER+/ER- and PR+/PR- groups.