2553
Time-Dependent Diffusion MRI for the Breast to Differentiate between Benign and Malignant Lesions1Department of Radiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 2Philips Healthcare, Guangzhou, China, 3Philips Healthcare, Beijing, China
Synopsis
Keywords: Breast, Breast, Tumor, Diffusion, OGSE, PGSE
Defining the nature of benign and malignant breast tumors is essential to reduce unnecessary biopsies of benign tumors. Magnetic resonance imaging (MRI) is the most sensitive imaging modality for evaluating breast carcinoma lesions, but its specificity is relatively low. This study investigated the significance of the parameters for the differential diagnosis of mammary mass using time-dependent diffusion MRI. Results showed significant cell size differences between benign and malignant space-occupying lesions, with high accuracy (85.7%), specificity (83.3%), sensitivity (87.5%) and AUC (0.823). This provides a new direction for noninvasively differentiating benign and malignant lesions of the mammary gland.Introduction
Breast soft tissue lesions are common and are broadly categorized into either malignant or benign tumor lesions. Breast cancer is the most common malignancy in the female population, and the incidence rate is increasing [1, 2]. Clarifying the nature of the tumor is critical for selecting therapeutic strategies and reducing the number of unnecessary biopsies performed on benign tumors [3].Magnetic resonance imaging (MRI) is the most sensitive imaging modality for evaluating breast carcinoma lesions, but its specificity is relatively low [4]. Diffusion-weighted imaging improves diagnostic accuracy in conventional 3.0-T breast MR imaging [5]. However, it may not reflect the microstructure of biological tissue such as the intra- and extra-cellular space, cell size, permeability, and intrinsic diffusivity [6]. More advanced imaging techniques are urgently needed to represent the microstructure of tumors. Time-dependent diffusion MRI reveals and correlates the time-dependent diffusion of restricted water molecules to the parameters of specific microstructures [7]. Previous studies have shown that time-dependent diffusion MRI is sensitive to microscopic pathologic characteristics in tumors on scales close to or even smaller than a single cell [8].
Therefore, this study aimed to noninvasively differentiate benign and malignant mammary tumors for clinical use based on tumor microstructure-represented parameters of time-dependent diffusion MRI.
Methods
Participants: A total of 28 consecutive patients with a clinical suspicion of breast space occupying were prospectively recruited at the Sun Yat-sen Memorial Hospital of Sun Yat-sen University from January 2022 to September 2022. Ethics committee approval was granted by the hospital research ethics board. Written informed consent was obtained from all participants to undergo time-dependent diffusion MRI in addition to standard-of-care multiparametric MRI. All patients underwent biopsies for clinical diagnosis for further group subdivision.MRI Data acquisition and Preprocessing: Structural time-dependent diffusion MRI technique requires the acquisition of diffusion MRI signals at varying diffusion times by using a combination of oscillating gradient spin-echo (OGSE) and pulsed gradient spin-echo (PGSE) sequences to capture the diffusion time dependency in different microstructural compositions. All scans were performed with a 3.0 T MRI scanner (Ingenia; Philips Healthcare, the Netherlands). OGSE sequence parameters: 50Hz (effective diffusion time = 10msec, two cycles, b = 100, 120, 150 and 170 sec/mm2) and 25Hz (effective diffusion time = 5msec, one cycle, b = 100, 200,500 and 600 sec/mm2). PGSE sequence parameters: effective diffusion time = 10 msec, b = 250, 500, 750 and 1000 sec/mm2. The following parameters were used for both sequences: three diffusion directions; repetition time/echo time (4740 msec/117 msec); field of view (250×250); in-plane resolution (100×100); number of sections (8); and section thickness (4 mm).
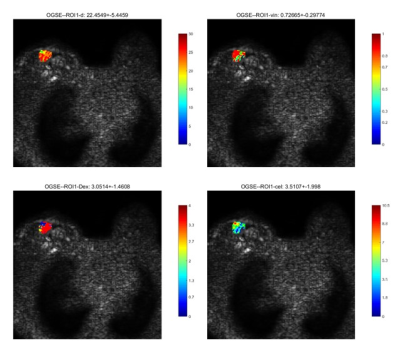
Image Analysis: The time-dependent diffusion MRI data were fitted using a two-compartment model with impermeable spheres according to the imaging microstructural parameters using limited spectrally edited diffusion (IMPULSED). Fitting was performed using the least square curve fitting in Matlab 2020b. Cell diameter, intracellular volume fraction, extracellular diffusivity and cell density were estimated (Figure 1).
Statistical analysis: The measurements for cell diameter, intracellular volume fraction, extracellular diffusivity and cell density at 55 Hz and 25 Hz were averaged over the manually delineated lesion regions of interest. The differences of parameters between malignant and benign tumor lesions were compared using one-way variance analysis followed by a comparative t-test. The correlations between the microstructural parameters were assessed by linear regression. The diagnostic efficacy of time-dependent diffusion MRI for benign and malignant tumors was evaluated with sensitivity, specificity, accuracy, and area under the receiver operating characteristic curve (AUC).
Sequences and post-processing tools were designed by local Philips clinical scientists as co-authors of this study.
Results
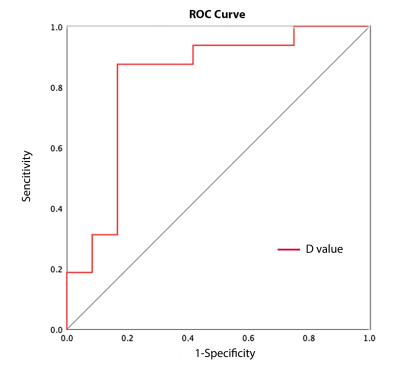
According to the pathological results, 12 patients were with benign breast tumor and the other 16 were with malignant breast tumor. Among the parameters derived from OGSE of benign and malignant breast tumors (Table 1), the mean cell diameter of benign breast lesions was significantly smaller comparing to that of malignant ones (19.85±3.17 vs. 23.70±2.89, P = 0.002). Additionally, intracellular volume fraction, extracellular diffusion rate and cell density showed no statistical differences between two groups. The cell diameter value for differentiating benign and malignant tumors of breast obtained a diagnostic performance with sensitivity (87.5%), specificity (83.3%), accuracy (85.7%) and AUC (0.823) (Table 2). Receiver Operating Characteristic (ROC) curve of cell diameter value is shown in Figure 2.Discussion & Conclusion
The time-dependent diffusion MRI technique is proposed to assess the microstructure of breast carcinoma. These time-dependent diffusive parameters, especially cellular parameters, have good accuracy in distinguishing the nature of the tumor. Although there were no statistical differences between benign and malignant tumors in terms of intracellular volume fraction, extracellular diffusion rate and cell density, the mean value of cell diameter of benign tumors was significantly smaller than that of malignant tumors. Time-dependent diffusion MRI using cell diameter parameter has high accuracy, specificity, sensitivity and AUC for the diagnosis of benign and malignant tumors. It has some potential clinical significance.The mean value of cell diameter of benign breast tumors is significantly smaller compared with malignant ones. Time-dependent diffusion MRI has high accuracy, specificity, sensitivity, and AUC in distinguishing between benign and malignant tumors. Therefore, it may provide a new direction for the noninvasively clinical diagnosis.
Acknowledgements
None.References
[1] Haynes B, Sarma A, Nangia-Makker P, Shekhar MP. Breast cancer complexity: implications of intratumoral heterogeneity in clinical management. Cancer Metastasis Rev 2017; 36:547-55. 10.1007/s10555-017-9684-y.
[2] Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008e2030): a population-based study. Lancet Oncol 2012;13:790e801.
[3] Paepke S., Metz S., Brea Salvago A., Ohlinger R. Benign Breast Tumours—Diagnosis and Management. Breast Care. 2018;13:403–412. doi: 10.1159/000495919.
[4] DeMartini W., Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Topics in Magnetic Resonance Imaging. 2008;19(3):143–150. doi: 10.1097/RMR.0b013e31818a40a5.
[5] Wu P., Cui L., Guo B. H., Wang Y. C., Cui J. S. Values of minimal apparent diffusion coefficient, difference between ratios of apparent diffusion coefficients, and dynamic contrast-enhanced magnetic resonance imaging features in diagnosing breast ductal carcinoma in situ with microinvasion. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41(6):737–745. doi: 10.3881/j.issn.1000-503X.11066.
[6] Kim J. J., Kim J. Y., Suh H. B., et al. Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. European Radiology. 2022;32(2):822–833. doi: 10.1007/s00330-021-08166-4.
[7] Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing meso- scopic structural universality with diffusion. Proc Natl Acad Sci U S A 2014;111(14):5088–5093.
[8] Reynaud O, Winters KV, Hoang DM, Wadghiri YZ, Novikov DS, Kim SG. Pulsed and oscillating gradient MRI for assessment of cell size and extracellular space (POMACE) in mouse gliomas. NMR Biomed 2016;29(10):1350–1363.
Figures