2549
Factors Affecting Cancer Detectability on Standalone Diffusion Weighted Imaging for Non-contrast Breast Cancer Screening1Radiology, University of Washington, Seattle, WA, United States, 2Radiology, Pusan National University Hospital, Busan, Korea, Republic of, 3Bio Engineering, University of Washington, Seattle, WA, United States, 4Philips Healthcare, Bothell, WA, United States, 5Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States
Synopsis
Keywords: Breast, Diffusion/other diffusion imaging techniques, Diffusion Weighted Imaging
Diffusion weighted imaging (DWI) is emerging as a viable tool for non-contrast MRI breast cancer screening, but it is unclear what factors on DWI impact lesion detectability. In this prospective clinical trial, we evaluated lesion and imaging factors that affected cancer detection. Cancers were overall more detectable at higher b=1200 vs b=800 s/mm2, but the background parenchymal signal (BPS) impacted cancer visibility at the higher b value. Cancer histologic type also impacted detectability on DWI. Overall, our findings suggest that interpretation at higher b values and further technical refinements to reduce appearance of BPS may help improve DWI sensitivity.Introduction
Diffusion weighted imaging (DWI) is a fast, widely available MRI technique that can demonstrate breast malignancies without the need for administration of exogenous contrast, making it a promising alternative to dynamic contrast enhanced (DCE)-MRI for screening applications. To evaluate the clinical utility for non-contrast screening in breast, it is critical to understand the underlying factors affecting cancer detectability on DWI. While several studies have investigated the impact of technical factors (b values, image quality) and histology on cancer conspicuity on DWI [1-6], there are limited data describing the influence of other patient factors. Of particular interest is the impact of background parenchymal signal (BPS) on DWI, which refers to the intensity of signal in the normal fibroglandular tissue due to T2-shine through and/or hindered diffusion [7-8]. BPS is somewhat analogous to background parenchymal enhancement (BPE) on DCE-MRI, which itself has variably been shown to affect conventional screening MRI performance [9]. The purpose of this study was to evaluate factors affecting breast cancer detectability on DWI with the added context of assessing BPS at different b-values.Materials and Methods
Subjects: In this IRB approved prospective DWI trial (ClinicalTrials.org NCT03607552) in women with dense breasts, enrollment was conducted in two phases: A) July 2018 – June 2019 for patients with known biopsy-proven breast cancer and B) November 2020 – October 2022 for patients with a BI-RADS 4/5 lesion prior to undergoing biopsy.MRI Acquisition: Imaging was performed on a 3T clinical scanner (Achieva, Philips Healthcare, Best, Netherlands) using a 16-channel breast coil. The breast MRI protocol included: T2-weighted, DWI, and DCE-MRI sequences. Phase A: DWI was acquired with TR/TE=3500/80 ms, FOV = 360x360 mm2, voxel size = 1.8×1.8×4 mm3, NSA=2, multiband sense factor 2, SPAIR with gradient reversal fat suppression, b=0, 100, 800, 1500, 2500 s/mm2, 30 slices, and 3:33 min scan time. Phase B: All parameters were same except b values = b=0, 100, 800, 1200 s/mm2, TR/TE = 3500/65 ms, 40 slices, and 2:43 min scan time.
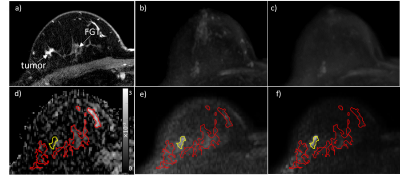
Image Analysis: BPS was qualitatively scored by a radiologist, at both b= 800 and 1200 s/mm2, using a 4-point scale (1: minimal, 2: mild, 3: moderate, 4: marked) and evaluating maximum intensity projection images (MIPs) to characterize the whole breast. For the Phase A cohort, MIPs were created from synthesized b=1200 s/mm2 images using vendor software (PARADISE workstation, Philips Healthcare). Quantitative image analysis was performed using custom software developed in MATLAB (Mathworks, Natick, MA). ADC maps were calculated using b=0 and 800 s/mm2 following EUSOBI guidelines [10]. Cancers were segmented on b=800 s/mm2 images and normal fibroglandular tissue was segmented on b=0 s/mm2 and propagated to higher b value images (Fig1). Cancer detectability was measured on diffusion-weighted images using relative contrast (RC), defined as
RC = ((μc - μn)/μn) × 100
Where µc and µn represent mean signal intensity of cancer and fibroglandular tissue respectively. RC was calculated at b=800 and 1200 s/mm2.
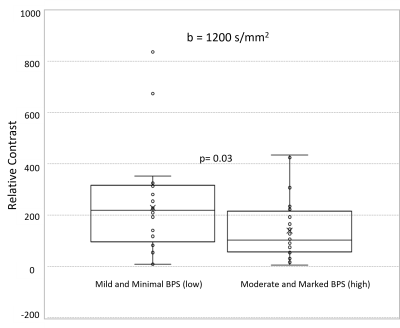
Statistical Analysis: BPS was dichotomized as low (mild and minimal) and high (moderate and marked) for analysis. RC and BPS at different b values were compared using Wilcoxon signed rank and chi-square test respectively. Subgroup analyses exploring the association of cancer detectability (RC800 and RC1200) with patient and tumor factors were performed using Wilcoxon rank sum test.
Results
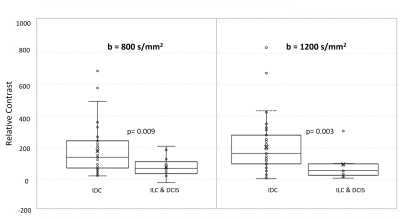
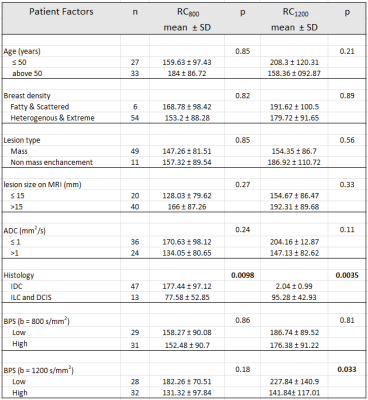
Overall, DWI scans in 60 women with dense breasts and pathologically confirmed cancers (30 Phase A, 30 Phase B) were evaluated qualitatively and quantitatively. Cancer histologies included invasive ductal carcinoma (n=47), invasive lobular carcinoma (n=6), and ductal carcinoma in situ (n=7). Mean ADC0-800 for cancers and normal tissue were 1.01 ± 0.38 mm2/s, and 2.05 ± 0.67 mm2/s, respectively. Tumor detectability at b=800 s/mm2 was significantly lower than at b=1200 s/mm2 (mean RC800 =155.65 vs RC1200 =180.32, p=0.03). More subjects exhibited marked BPS at b=800 s/mm2 (n=16) than at b=1200 s/mm2 (n=11), though the overall difference in BPS between b-values was not statistically significant (p=0.6). Of all patient and lesion factors evaluated, only BPS at b=1200 s/mm2 (low vs high; p=0.03) and cancer histologic type (IDC vs other cancers; p=0.009 on b=800 s/mm2 and p=0.003 on b=1200 s/mm2) were significantly associated with cancer detectability. (Figs 2, 3, 4)Discussion and Conclusion
Our results show that breast cancer detectability on non-contrast DWI is better at b=1200 s/mm2 compared to b=800 s/mm2, which is widely used as the standard for breast DWI acquisition. Reduced detectability at b=800 s/mm2 may be due to the higher levels of BPS observed versus b=1200 s/mm2. However, even at b=1200 s/mm2, residual BPS impacts cancer detectability. Furthermore, cancer histologic type also impacts detectability on DWI, with invasive ductal carcinomas demonstrating higher detectability than other cancers, which agrees with prior literature [6]. In summary, our findings demonstrate that for utilization of DWI as a standalone non-contrast technique for breast cancer detection, interpretation at higher b-values (>800 s/mm2) with extra consideration for presence of background parenchymal signal may optimize sensitivity.Acknowledgements
We would like to acknowledge our funding sources R01CA207290, Safeway Foundation and in-kind research support from Philips Healthcare.References
1) DelPriore MR, et al. Breast Cancer Conspicuity on Computed Versus Acquired High b-Value Diffusion-Weighted MRI. Acad Radiol. 2021 Aug;28(8):1108-1117.
2) O'Flynn EA, et al. Evaluating the diagnostic sensitivity of computed diffusion-weighted MR imaging in the detection of breast cancer. J Magn Reson Imaging. 2016 Jul;44(1):130-7.
3) Pinker K, et al. Diffusion-Weighted Imaging with Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone Parameter: Comparison with Dynamic Contrast-Enhanced and Multiparametric Magnetic Resonance Imaging. Investigative Radiology: October 2018 - Volume 53 - Issue 10 - p 587
4) Whisenant JG, et al. Factors Affecting Image Quality and Lesion Evaluability in Breast Diffusion-weighted MRI: Observations from the ECOG-ACRIN Cancer Research Group Multisite Trial (A6702). J Breast Imaging. 2020 Dec 24;3(1):44-56.
5) McDonald ES, et al. Performance of DWI as a Rapid Unenhanced Technique for Detecting Mammographically Occult Breast Cancer in Elevated-Risk Women with Dense Breasts. AJR Am J Roentgenol. 2016 Jul;207(1):205-16
6) Amornsiripanitch N, et al. Diffusion-weighted MRI for Unenhanced Breast Cancer Screening. Radiology. 2019 Dec;293(3):504-520.
7) Hahn SY, et al. Analysis of factors influencing the degree of detectability on diffusion-weighted MRI and diffusion background signals in patients with invasive breast cancer. Medicine (Baltimore). 2016 Jul;95(27).
8) Kim JJ, Kim JY. Fusion of high b-value diffusion-weighted and unenhanced T1-weighted images to diagnose invasive breast cancer: factors associated with false-negative results. Eur Radiol. 2021 Jul;31(7):4860-4871. doi: 10.1007/s00330-020-07644-5. Epub 2021 Jan 14. PMID: 33443601.
9) Liao GJ, et al. Background parenchymal enhancement on breast MRI: A comprehensive review. J Magn Reson Imaging. 2020 Jan;51(1):43-61.
10) Baltzer P, et al; EUSOBI international Breast Diffusion-Weighted Imaging working group. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol. 2020 Mar;30(3):1436-1450.
Figures