Joo Han1, Justin D. Sprick2,3, Lisa C. Krishnamurthy1,4, Serena Song1, and Venkatagiri Krishnamurthy1,5
1Center for Visual and Neurocognitive Rehabilitation, Atlanta VAMC, Decatur, GA, United States, 2Division of Renal Medicine, Department of Medicine, Emory University, Atlanta, GA, United States, 3University of North Texas Health Science Center, Denton, TX, United States, 4Department of Physics & Astronomy, Georgia State University, Atlanta, GA, United States, 5Division of Geriatrics and Gerontology, Department of Medicine, Emory University, Atlanta, GA, United States
Synopsis
Keywords: Signal Modeling, fMRI (task based)
The limitation of estimating Dynamic Cerebral Autoregulation
(dCA) using Transcranial Doppler ultrasound is the lack of whole-brain estimation
capabilities. In this study, the data acquisition of whole-brain task-fMRI scan
during a novel Passive Cyclical Leg Raise (PCLR) task allows us to induce blood
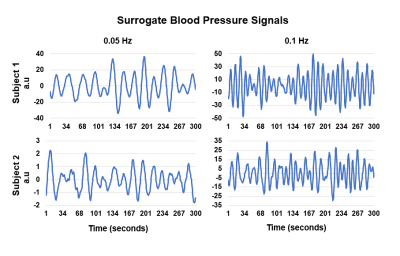
pressure (BP) changes. A surrogate BP signal was acquired from the brain stem
and depicts expected fluctuations based on the PCLR-task design. The whole-brain
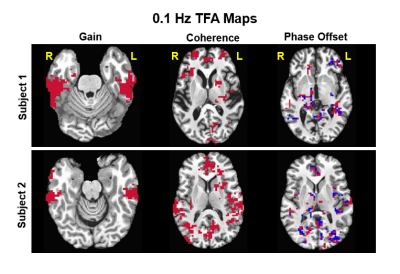
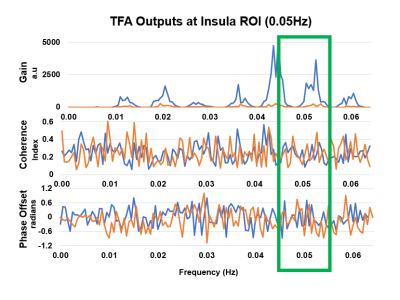
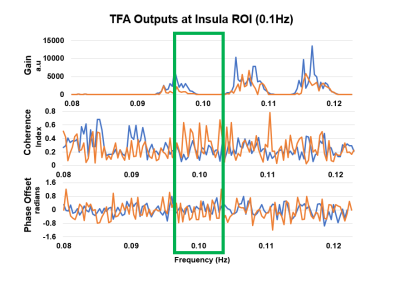
dCA was estimated using voxel-wise TFA to obtain the transfer gain, coherence,
and phase-offset. These results show similar significant brain regions but with
distinct values based on the participant’s varying level of constitution.
Acknowledgements
We thank Dr. Keith McGregor for his assistance in data collection.References
1. R. Zhang, J. Zuckerman, C. Giller, and B. Levine. Transfer function analysis of dynamic cerebral autoregulation in humans. American Journal of Physiology-Heart and Circulatory Physiology, 274(1), pp. H233-H24. 1998. doi/10.1152/ajpheart.1998.274.1.H233.
2. Panerai RB, Brassard P, Burma JS, et al. Transfer function analysis of dynamic cerebral autoregulation: a CARNet white paper 2022 update. Journal of Cerebral Blood Flow & Metabolism, 0(0), 2022. doi:10.1177/0271678X221119760
3. V. Macefield, L. Henderson. Identification of the human sympathetic connectome involved in blood pressure regulation. NeuroImage, 202, 2019. doi.org/10.1016/j.neuroimage.2019.116119.
4. J. Whittaker, I. Driver, M. Venzi, M. Bright, and K. Murphy. Cerebral Autoregulation Evidenced by Synchronized Low Frequency Oscillations in Blood Pressure and Resting-State fMRI. Front. Neursci., 2019. doi.org/10.3389/fnins.2019.00433.