2539
Bayesian Longitudinal Tensor Response Regression to model task-fMRI based neuroplasticity in post-stroke aphasia1Dept. of Medicine, Emory University, Atlanta, GA, United States, 2Atlanta VA Medical Center, Decatur, GA, United States, 3Dept. of Biostatistics, Emory University, Atlanta, GA, United States, 4Dept. of Biostatistics, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Keywords: Data Analysis, fMRI (task based), Neuroplasticity
Stroke is inherently complex due to the heterogeneity in lesion location, size in addition to other clinical comorbidities. Further, longitudinal three-dimensional fMRI datasets add more complexity towards estimating sensitive and robust biomarkers of neuroplasticity. In this study we propose an innovative extension of Bayesian Tensor Response Regression (BTRR) approach to estimate neuroplasticity that is more sensitive, accurate and reliable compared to traditional voxel-wise approach. Results from our longitudinal aphasia-treatment study not only show that the BTRR approach is more superior but is also able to derive plasticity estimates that are sensitive to treatment differences that are subject and time-point specific.METHODS: MPRAGE and overt task-fMRI images were acquired at the baseline, 2-weeks and 3-month post-treatment from fourteen English speaking aphasia (post-stroke>=6 months) participants. Overt task-fMRI paradigm involved generating an exemplar for a given semantic category. Images were slice time corrected, motion corrected (including speech-induced task-correlated motion4) and warped into MNI space using chimera spatial normalization. Voxel-wise hemodynamic response function was estimated using deconvolution followed by quantification of the z-transformed area under the curve (ZAUC). All subjects underwent language therapy in three phases consisting of picture naming and category generation. Subjects were randomized into no gesture (N=7) or gesture (N=7) corrective treatments. Unlike the no gesture corrective treatment, intentional gesture treatment involved left hand initiation and circular motion when orally correcting incorrect responses5. The novel BLTRR modeling was implemented on the ZAUC data and it featured – (i) at the population level, intercept term that can be assigned a tensor structure, time-varying effects to capture longitudinal changes and time-invariant effects, (ii) at the individual subject level, random intercept term to capture baseline deviations and time slopes to capture variations in the longitudinal trajectory. Finally, we also included random residual errors assumed to be independently distributed. Instead of treating each voxel as a separate unit, the voxel specific coefficients were modeled using a low-rank PARAFAC decomposition that pooled data across neighboring voxels to estimate a given voxel-specific coefficient. For the prior specifications on the tensor margins, we utilized a parametric low-rank structure which is complementary to the advantages of spatial smoothing. The posterior distribution was used for estimation under a Bayesian framework, resulting in data-adaptive correlation estimates that were allowed to vary over brain regions. Within the Bayesian framework, we used joint credible regions for inference and feature selection that recognized the correlations in the posterior distribution and thereby incorporating a naturally in-built multiplicity adjustment mechanism.
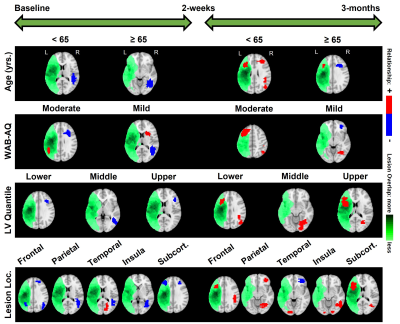
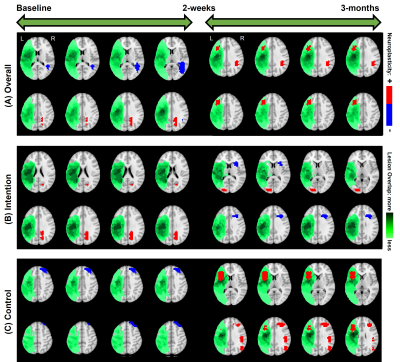
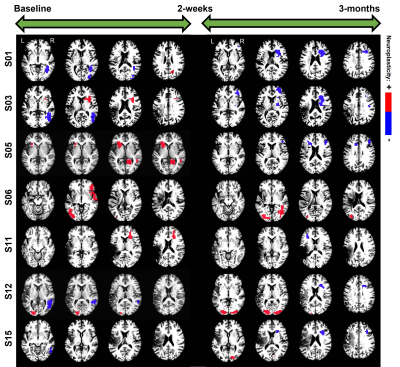
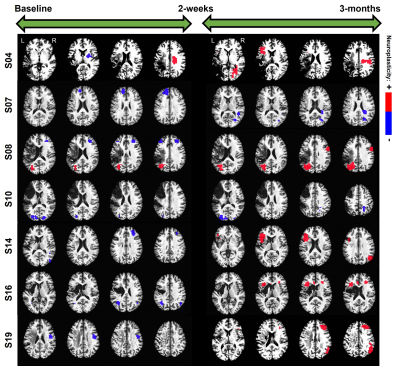
RESULTS: In terms of the chosen covariates (i.e., clinical factors) of interest, from Figure-1 it is interesting to note that across all the covariates, the effects of language therapy (irrespective of standard or intention therapy) had pronounced positive effects on semantic category member generation. While it is worthwhile to identify that lesion location and lesion volume influence the long-term plasticity, our results indicate that age is a critical factor in harnessing positive plastic changes. That is, participants younger than 65 have more rehabilitation potential to benefit from treatment-specific plastic changes, and those older than 65 may need more tailored and additional treatments to gain long-term plastic changes. From Figure-2, we observe that the model provided consistent positive neuroplastic estimates for long-term changes when all participants were pooled together irrespective of specific (i.e. standard or intention) therapy. Further, when the participants were separated based on the type of treatment, our novel modeling approach was able to identify unique biomarkers for treatment-specific neuroplastic changes. In terms of neuroplasticity maps, the standard voxel-wise regression failed to detect any significant changes after multiplicity adjustments. From Figures 3 and 4, our results not only show that our novel approach has the potential to generate individualized maps, but also such individualized results show consistent trends in positive/negative plastic changes that are treatment and time point specific.
DISCUSSION: The importance of the tensor-based approach for the analysis of longitudinal Aphasia data becomes clearly evident from superior out-of-sample predictive performance over voxel-wise methods and given the fact that the voxel-wise approach is unable to infer any significant neuroplasticity changes after multiplicity adjustments. The estimated neuroplasticity changes are not only in line with previously observed behavioral changes5 from the same subject group, but also clinically pragmatic considering that we accounted for critical clinical factors.
CONCLUSION: Robust task-specific biomarkers of treatment-neuroplasticity may aide in improved understanding of the underlying neurobiological mechanism that could be useful in treatment planning tailored to participant’s baseline clinical profile.
Acknowledgements
No acknowledgement found.References
1. M. Pekna, M. Pekny, and M. Nilsson. Modulation of neural plasticity as a basis for stroke rehabilitation. Stroke, 43(10), pp. 2819–2828, 2012. doi:10.1161/STROKEAHA.112.654228.
2. S. M. Wilson and S. M. Schneck. Neuroplasticity in post-stroke aphasia: a systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiology of Language, 2(1), pp. 22–82, 2021. doi:10.1162/nol_a_00025.
3. R. Guhaniyogi and D. Spencer. Bayesian tensor response regression with an application to brain activation studies. Bayesian Anal., 16(4), pp. 1221–1249, 2021. doi:10.1214/21-BA1280.
4. V. Krishnamurthy, L. C. Krishnamurthy, M. L. Meadows, M. K. Gale, B. Ji, K. Gopinath, and B. Crosson. A method to mitigate spatio-temporally varying task-correlated motion artifacts from overt-speech fMRI paradigms in aphasia. Hum. Brain Mapp., 42(4), pp. 1116–1129, 2021. doi:10.1002/hbm.25280.
5. L. J. Altmann, A. A. Hazamy, P. J. Carvajal, M. Benjamin, J. C. Rosenbek, and B. Crosson. Delayed stimulus-specific improvements in discourse following anomia treatment using an intentional gesture. J. Speech Lang. Hear. Res., 57(2), pp. 439–454, 2014. doi:10.1044/1092-4388(2013/12-0224).
Figures