2537
Multi-echo EPI more effectively boosts BOLD sensitivity than sequence optimization in orbitofrontal cortex1Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Centre de Résonance Magnétique des Systèmes Biologiques, CNRS‐University Bordeaux, Bordeaux, France, 3Max Planck University College London Centre for Computational Psychiatry and Ageing Research, London, United Kingdom
Synopsis
Keywords: Data Acquisition, fMRI
Multi-echo fMRI can boost BOLD sensitivity relative to conventional single-echo fMRI, especially in high-susceptibility brain regions like orbito-frontal cortex (OFC). Another option is to optimise slice-tilts, z-shimming and k-space traversal to minimise susceptibility effects. In this study, we sought to determine if multi-echo EPI, which requires the use of parallel imaging to achieve reasonable echo times, would remain optimal in OFC when compared to an OFC-optimised single echo alternative. The relative performance is quantified via BOLD contrast-to-noise ratio and an additional comparison is made by incorporating the TE Dependent ANAlysis (TEDANA) denoising approach. Multi-echo increased BOLD CNR, particularly following denoising.
Introduction
Orbitofrontal cortex (OFC) is involved in many cognitive tasks including memory, decision-making and emotional/social behaviours1. Unfortunately, susceptibility-induced B0-field inhomogeneity in this region accentuates signal dropout, and distortion, in 2D gradient-echo echo-planar imaging (EPI). In comparing a range of different sequence options, Kirilina et al.2 found that a multi-echo acquisition3 could combat signal dropout and bolster BOLD sensitivity, specifically in the OFC – an improvement preserved at the group level. However, this was relative to standard 2D-EPI. A well-established alternative for recovering BOLD sensitivity is to integrate slice tilts and z-shimming gradients specifically optimised for the OFC region4. In this study, we sought to determine if multi-echo EPI, which requires parallel imaging to achieve reasonable echo times (TE), would remain optimal in OFC relative to an OFC-optimised single-echo alternative.Material & Methods
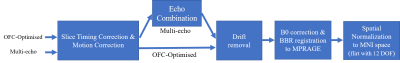
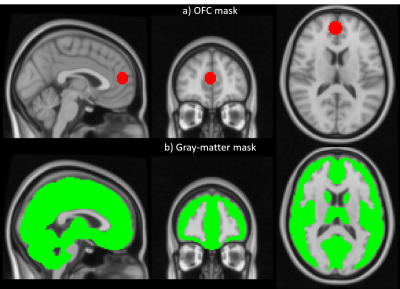
Data acquisition: 9 participants were scanned at 3T (Siemens Prisma) using a 64 channel coil. Time series data with 3mm isotropic resolution were acquired with both a multi-echo and an OFC-optimised protocol (Table 1) while the participants engaged in a decision-making task. In brief, the OFC-optimised protocol had a TE of 30ms, a slice tilt of −30° and a z-shimming gradient moment of -1.4 mt/m*ms to mitigate signal dropout4,5. The multi-echo acquisition comprised three echoes with TE of 17.5ms, 35.6ms and 53.7ms. This extended the repetition time, TR, (3.84s relative to 3.36s) and necessitated an in-plane acceleration factor of 2. Matching the total acquisition time led to 150 and 172 volumes for the multi-echo and OFC-optimised protocols respectively. B0-field mapping and a T1-weighted MPRAGE were also acquired.Data processing: Two participants were excluded during quality assessment due to excessive motion (exceeding the voxel dimension) during data acquisition. All pre-processing and analyses were performed using FSL6 and MATLAB (Figure 1). First, slice-timing and motion correction were applied to the OFC-optimised and multi-echo data. Next, the multi-echo data were combined either as a simple average over TE7, or by using the TE-Dependent ANAlysis (TEDANA) denoising approach8,9. Finally, drift removal with a cut-off frequency of 0.01 Hz and distortion correction using the B0-field mapping data were applied to both datasets. The BOLD sensitivity of each dataset was estimated in grey matter (GM) and the OFC for each participant in native space. Both regions of interest (ROIs) were defined in MNI space and transformed to native space via inverse transformation (Figure 1). The OFC was defined according to Kirilina et al.2 as a spherical ROI, of 10 mm diameter, centred on coordinates (3,51,−14) (Figure 2a). The GM mask was defined as those voxels with > 50% probability of being GM according to a canonical tissue probability map (Figure 2b).
Analysis: CNRBOLD was calculated using equation (1)7, where Si is the voxel’s signal amplitude at TEi and n is the number of echoes. Following TEDANA denoising, CNRBOLD was calculated using the average TE.
$$CNR_{BOLD}=\frac{mean(\frac{\sum_1^nTE_{i}.S_{i}}{n})}{std(\frac{\sum_1^nS_{i}}{n})} \;\;\;\;\;\;\;\;\;\;\;\;\;(1)$$
Results
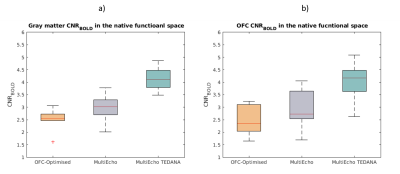
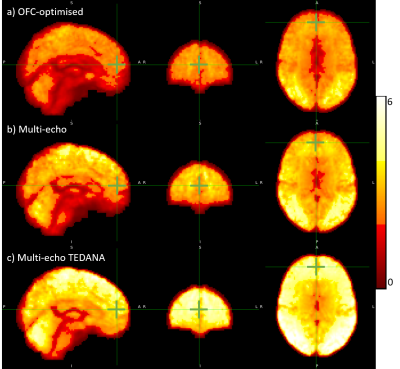
In GM, the median CNRBOLD across the cohort was 19% higher for the multi-echo relative to the OFC-optimised data (3.02 and 2.35 respectively). However, the multi-echo data also had a larger inter-quartile range (IQR of 0.60, versus 0.26 for OFC-optimised data). TEDANA denoising further increased the CNRBOLD (median±IQR=4.12±0.69). The median CNRBOLD was reduced for both sequences in the OFC, but remained 16% higher for the multi-echo data (2.35, versus 2.73 for OFC-optimised). However, in this ROI the IQR was more comparable (1.12, versus 1.07 for OFC-optimised). The TEDANA denoising further increase CNRBOLD (median±IQR=4.14±0.85). Figure 4 shows the group-level CNRBOLD map for each approach. The green cursor indicates the centre of the OFC ROI. This qualitative assessment aligns with the quantitative assessment. Multi-echo offers a modest gain over the OFC-optimised approach, with further gains following TEDANA denoising.Discussion
This study builds on previous work that showed multi-echo acquisitions can increase BOLD sensitivity in the OFC by acquiring data with shorter TE and reduced signal dropout. Here we show that this is the case even when compared to a 2D-EPI acquisition optimised for imaging the OFC, and particularly so when the multi-echo nature of the data is exploited for denoising to detect and remove TE-independent (i.e. non-BOLD) components8. This gain in BOLD sensitivity occurs despite the use of in-plane acceleration necessary to acquire multiple echoes with sufficiently short inter-echo spacing and TR to not overly degrade temporal efficiency. This acceleration will incur a √2 reduction in SNR, plus any g-factor penalty, and leads to greater sensitivity to motion occurring between the acquisition of calibration data and the ongoing time series data. However, it is also worth noting that the shorter readout used with in-plane acceleration provides the benefit of reducing any susceptibility-induced distortion, which will be particularly beneficial in the OFC.In conclusion, the multi-echo approach continued to outperform single-echo 2D-EPI in the OFC even after incorporating optimal slice tilt and z-shim settings for this region. The benefits were further amplified, across the entire GM, by exploiting the multi-echo nature of the data to perform denoising.
Acknowledgements
The Wellcome Trust funded the “Neuroscience in Psychiatry Project” (NSPN). All NSPN members were supported by the Wellcome Strategic Award, ref. 095844/7/11/Z. The Max Planck – UCL Centre for Computational Psychiatry and Aging is a joint initiative of the Max Planck Society and UCL. M.M. received support from the National Institute for Health Research (NIHR) UCLH Biomedical Research Centre. The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome [203147/Z/16/Z]. This research was funded in whole, or in part, by the Wellcome Trust [203147/Z/16/Z].References
1. Rolls ET. The functions of the orbitofrontal cortex. Brain and cognition. 2004 Jun 1;55(1):11-29.
2. Kirilina, E., Lutti, A., Poser, B.A., Blankenburg, F. and Weiskopf, N., 2016. The quest for the best: The impact of different EPI sequences on the sensitivity of random effect fMRI group analyses. Neuroimage, 126, pp.49-59.
3. Poser, B.A. and Norris, D.G., 2009. Investigating the benefits of multi-echo EPI for fMRI at 7 T. Neuroimage, 45(4), pp.1162-1172.
4. Weiskopf, N., Hutton, C., Josephs, O. and Deichmann, R., 2006. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. Neuroimage, 33(2), pp.493-504.
5. Volz, S., Callaghan, M.F., Josephs, O. and Weiskopf, N., 2019. Maximising BOLD sensitivity through automated EPI protocol optimisation. Neuroimage, 189, pp.159-170.
6. Jenkinson, M., Beckmann, C.F., Behrens, T.E., Woolrich, M.W. and Smith, S.M., 2012. Fsl. Neuroimage, 62(2), pp.782-790.
7. Kettinger, Á., Hill, C., Vidnyánszky, Z., Windischberger, C. and Nagy, Z., 2016. Investigating the group-level impact of advanced dual-echo fMRI combinations. Frontiers in neuroscience, 10, p.571.
8. Kundu, P., Inati, S.J., Evans, J.W., Luh, W.M. and Bandettini, P.A., 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage, 60(3), pp.1759-1770.
9. DuPre, E., Salo, T., Ahmed, Z., Bandettini, P.A., Bottenhorn, K.L., Caballero-Gaudes, C., Dowdle, L.T., Gonzalez-Castillo, J., Heunis, S., Kundu, P. and Laird, A.R., 2021. TE-dependent analysis of multi-echo fMRI with* tedana. Journal of Open Source Software, 6(66), p.3669.
Figures