2534
Spatial-temporal reconstruction using UNFOLD in looping-star silent fMRI1EECS, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Synopsis
Keywords: Image Reconstruction, Brain
Looping star is a silent MRI pulse sequence that can be used for quantitative susceptibility mapping (QSM), T2*-weighted imaging and fMRI. However, the sparse radial sampling of looping-star limits its spatial and temporal resolution in fMRI studies. This work proposes a customized looping-star fMRI protocol and a spatial temporal reconstruction method that removes the undersampling artifact from the repeating sampling pattern and improves the temporal resolution and quality of the time course and activation map.Introduction
Functional magnetic resonance imaging (fMRI) has evolved into the dominant tool for noninvasively imaging human brain activity. However, the loud acoustic noise in MRI still remains a problem. For example, acoustic noise could cause discomfort and anxiety in patients, especially in certain groups like children or patients with dementia. Furthermore, acoustic noise is an additional confounding sensory stimulus [1] and can impact the blood-oxygen level dependent (BOLD) response as a function of both its loudness and duration.Looping-star [2] is a silent MRI pulse sequence that can be used in QSM, T2*-weighted imaging, and fMRI. The quiet feature makes it well-suited for pediatric MRI and auditory fMRI tasks. However, looping-star MRI suffers from the sparse radial sampling and low SNR, which limits its temporal and spatial resolution in fMRI. Due to the high undersampling factor, sometimes the volume TR must be increased to collect more k-space data and reduce undersampling artifacts at the cost of worse temporal resolution. In this work, we combined two methods to reduce the undersampling artifact and improve the temporal resolution. First, we apply our previously described model-based reconstruction [3] for overlapping echoes, which has less undersampling than the coherence resolved method [4]. Second, we use UNFOLD [5] to improve the temporal resolution by a factor of 2 and reduce the undersampling artifact due to the alternating sampling patterns.
Methods
Pulse sequence: Figure 1 shows the pulse sequence we used for experiments. Compared to the original looping-star fMRI protocol, we optimized some parameters to improve the robustness and performance of the pulse sequence. First, we used a 3D generalized golden-angle-based rotation [6] instead of random rotation to achieve a more uniform distribution of spokes in k-space. Secondly, we reduced the number of RF pulses to 23 with increased sampling time, which improves the spatial resolution from 3.75 mm to 3 mm. Thirdly, because higher spatial resolution requires more spokes in k-space, we increased the volume TR to 3.6 sec to reduce the undersampling artifact. The same sampling pattern is repeated every volume TR to minimize the impact of the sampling pattern on fMRI signals.Problem formulation: In looping-star MRI, gradient echoes are overlapped in the time domain due to multiple RF pulses. Therefore, we use the following signal model to account for multiple echoes:
$$s_j(t) = \int c_j(r) f(r) \sum_{l=1}^L \mathrm{e}^{-\textit{i} 2\pi k(r) \cdot t_l} dr,$$
where $$$c_j(r)$$$ is the sensitivity map of $$$j$$$th receiver coil, $$$f(r)$$$ is the continuous T2*-weighted object, and $$$L$$$ is the number of RF pulses that were applied before time $$$t_l$$$. This signal model fully models both echo-in and echo-out spokes and resolves the overlapping echo problems.
Reconstruction: We used CG-SENSE with a spatial 3D quadratic roughness regularizer for reconstruction. Each frame from the scan is reconstructed independently as a reference. For the spatial-temporal reconstruction, we first split every frame into two sub-frames with equal duration and separately reconstructed each sub-frame. Then we used UNFOLD to remove the undersampling artifact from the alternating sampling pattern.
Experiments: We compared our model-based image reconstruction (MBIR) with 1) lower temporal resolution, 2) higher temporal resolution before UNFOLD filtering, and 3) higher temporal resolution after UNFOLD filtering. In the fMRI study, participants watched a flashing checkerboard for multiple cycles (20s on and 20s off), and were required to tap their fingers while the checkerboard was on. The pulse sequences were programmed via TOPPE [7] and implemented on a GE UHP 3.0T scanner with a Nova 32RX head coil. Subjects gave informed consent under IRB approval.
Results
Fig. 2 shows the time series of a looping-star fMRI task. It can be seen that the undersampling artifact in (b) due to the repeating sampling pattern is significantly reduced after applying UNFOLD (c), leading to increased temporal resolution and reduced undersampling artifact.Fig. 3: (a) shows the FFT of the time course of a typical activated voxel. There is a significant high-frequency component because the undersampling artifact oscillates between adjacent frames. Before UNFOLD, the raw activation map (b) does not reflect the true brain activity due to the undersampling artifacts. After UNFOLD, even by only removing a single frequency component, the new activation map (c) shows higher correlation coefficients and recovered activities.
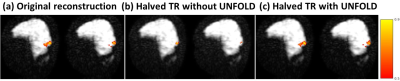
Fig. 4: Sagittal activation map for the visual stimulus. Improving the temporal resolution by a factor of 2 (from (a) to (b)) initially greatly reduces activation, due to the increased noise from the undersampling artifacts. However, after removing the undersampling artifact using UNFOLD, the activation map is improved back to the level of the original reconstruction, but with doubled temporal resolution.
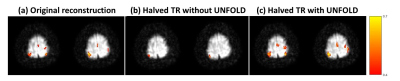
Fig. 5 shows the activation map for the finger-tapping tasks. Similar to the visual task, using UNFOLD recovers brain activities by reducing the undersampling artifact.
Conclusion
As demonstrated on both visual and motor tasks, the proposed method shows improvement in the temporal resolution and reduction of undersampling artifacts, at a negligible cost to temporal resolution from removing a single frequency component. For further study, more advanced spatial-temporal models can be explored to reduce undersampling artifacts for repeating and non-repeating sampling patterns.Acknowledgements
This work is supported in part by NIH Grants R01 EB023618.
References
[1] Peelle, J. E. (2014). Methodological challenges and solutions in auditory functional magnetic resonance imaging. Frontiers in neuroscience, 8, 253.
[2] Dionisio‐Parra, B., Wiesinger, F., Sämann, P. G., Czisch, M., & Solana, A. B. (2020). Looping star fMRI in cognitive tasks and resting state. Journal of Magnetic Resonance Imaging, 52(3), 739-751.
[3] Xiang, Haowei, Fessler, A., Jeffrey, Noll, C., Douglas. "Model-based Image Reconstruction in Looping-star MRI." ISMRM 2022
[4] Damestani, N. L., Lythgoe, D. J., Solana, A. B., Fernandez, B., Williams, S. C. R., Zelaya, F., & Wiesinger, F. Coherence-resolved Looping Star–improvements for silent multi-gradient echo structural and functional neuroimaging.
[5] Madore, B. (2004). UNFOLD‐SENSE: a parallel MRI method with self‐calibration and artifact suppression. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 52(2), 310-320.
[6] Chan, R. W., Ramsay, E. A., Cunningham, C. H., & Plewes, D. B. (2009). Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 61(2), 354-363.
[7] Nielsen, J. F., & Noll, D. C. (2018). TOPPE: A framework for rapid prototyping of MR pulse sequences. Magnetic resonance in medicine, 79(6), 3128-3134.
Figures
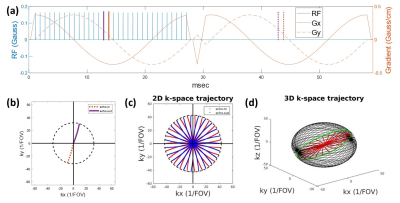
Figure 1: (a) A pulse sequence for a 2D plane of the 3D acquisition with one excitation/FID module and one GRE/data acquisition module (ramp-up & down gradient is required by TOPPE), the max slew rate for all modules (including ramps) is 5 mT/m/ms; (b) Illustration of overlapping echoes in GRE module: the echo-out signal from purple RF pulse overlaps the echo-in signal from orange RF pulse in time; (c)2D GRE k-space trajectory: odd number of spokes are used to generate more evenly distributed spokes; (d) 3D k-space trajectory using 3D generalized golden-angle.
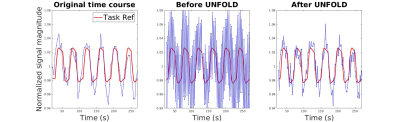
Figure2: fMRI time course from visual stimulus. Left to right: reconstruction with ~3.6 sec temporal resolution; reconstruction with ~1.8 sec temporal resolution without UNFOLD; reconstruction with ~1.8sec temporal resolution with UNFOLD. Due to the high undersampling rate, the middle figure shows significant signal oscillation from the different sampling trajectories when we reduce the time for each frame. After removing the undersampling artifact in the k-t domain using UNFOLD, the oscillations are mostly removed and temporal resolution is also improved.

Figure3 shows the FFT of a time course of a typical activated voxel. Before UNFOLD, the raw activation map does not reflect the true brain activity because the undersampling artifacts oscillate between adjacent frames. After UNFOLD, even only removing a single frequency component, the undersampling artifact is reduced such that the new activation map shows higher correlation coefficient and recovered activities.