2519
A machine learning model for identifying idiopathic central precocious puberty in girls based on medical images and clinical multi-parameters1Radiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China, 2Philips Healthcare, Shanghai, China
Synopsis
Keywords: Adolescents, Endocrine
Early identification of precocious puberty (PP) is important to guarantee the growth and development of children. The aim of this study was to propose a robust machine learning model that incorporate information from pituitary MRI images, carpal bone age, gonadal ultrasound, baseline sex hormone tests, and clinical information to identify idiopathic central precocious puberty (ICPP). The experiments show that the AUCs are 0.860, 0.862, and 0.866 respectively for the training set, internal validation sets, and external validation sets. The performance suggests that the presented model could be an alternative clinical approach.
Introduction
Precocious puberty (PP), if not intervened early, can seriously affect children's growth and development. The current gold standard for identifying idiopathic central precocious puberty (ICPP) and peripheral precocious puberty (PPP) is the gonadotropin-releasing hormone (GnRH) stimulation test1. However, this invasive test requires the injection of drugs and multiple blood collections, which may hinder the timely diagnosis of the disease due to poor cooperation from patients and families. We aim to develop diagnostic classification models by integrating pituitary MRI, carpal bone age, gonadal ultrasound, and routine clinical examinations to accurately diagnose ICPP.Methods
In this study, 492 female children diagnosed with precocious puberty were enrolled and their clinical data and medical images were retrospectively analyzed. All subjects were divided into the ICPP group (n=185) and the PPP group (n=307) based on the results of the GnRH stimulation test. Then, they were randomly divided into training and internal validation sets with a fixed ratio of 7.5:2.5 for each group. Moreover, 51 cases from another hospital were adopted as the external validation set. The pituitary MRI data were manually segmented (Figure 1) and the feature extraction was performed, and the intraclass correlation coefficient (ICC) of inter-observer and Intra-observer was applied to ensure the consistency of segmentation. Carpal bone age data were interpreted by artificial Intelligence using three different methods including TW3-RUS, TW3-Carpal, and CN05. Gonadal ultrasounds were recorded including bilateral ovarian volume, bilateral follicle volume, uterine volume, and presence or absence of endometrium. And clinical information was collected, including age, height, weight, BMI, baseline sex hormone (Luteinizing hormone, Follicle-stimulating hormone, Estradiol, Testosterone, Prolactin, Progesterone), and Tanner classification. Screening different optimal features using machine learning methods for building multiple diagnostic classification models (Table 1), and the Hosmer-Lemeshow (HL) test is employed to evaluate the overfitting of models. Moreover, receiver operating characteristic (ROC) curves and the Delon test were generated to evaluate and compare the predictive performance of these models.Results
Four predictive models were created, and all passed the HL test. The area under the curve (AUC) of the four models, such as the pituitary MRI radiomics model, integrated medical image model, basic clinical model, and integrated multiparameter model, in the training set was 0.668, 0.809, 0.792, and 0.860, respectively. In the internal validation set, they were 0.641, 0.777, 0.833, and 0.866. The results of the Delong test between the four models based on the complete internal data showed a statistical difference (P values <0.001) between the diagnostic ability of the integrated multiparametric model and the other three models (Figure2). Besides, the AUC of the integrated multiparameter model in the external validation set was 0.866 with the highest diagnostic efficiency.Discussion
The best diagnostic classification model developed by logistic regression was the integrated multiparameter model, which exhibited excellent diagnostic efficacy in the internal sets and robust reproducibility in the external sets. The low diagnostic efficacy of the pituitary MRI radiomics model may be attributed to the fact that the pituitary gland carries different hormonal information and is susceptible to artifacts in the images due to interference from surrounding structures2. Basic clinical information and baseline sex hormones alone were not sufficient to diagnose ICPP3. Finally, the reliable integrated multiparameter model proposed may replace the GnRH stimulation test to some extent, and its simplicity could help improve the diagnosis of ICPP and make it more acceptable to patients and families.Conclusion
In this study, we developed a reliable, and robust applicable machine learning model that relies on pituitary MRI images, carpal bone age, gonadal ultrasound, baseline sex hormone tests, and clinical information to identify ICPP. The integrated multiparameter model has good diagnostic performance with AUCs of 0.860, 0.862, and 0.866 in the training set, internal validation sets, and external validation sets, which can effectively identify ICPP without relying on the GnRH stimulation test. This study provides an alternative clinical approach that may relieve the distress and consequent family non-cooperation caused by the GnRH stimulation test.Acknowledgements
No acknowledgement found.References
- Stephen H Bradley, Neil Lawrence, et al. Precocious puberty. BMJ (Clinical research ed.). 2020 Jan 13;368:l6597
- Hongyang Jiang, Zhenyu Shu, et al. Noninvasive radiomics-based method for evaluating idiopathic central precocious puberty in girls. The Journal of international medical research. 2021 Feb;49(2).
- Quynh Thi Vu Huynh, Nguyen Quoc Khanh Le, et al. Development and Validation of Clinical Diagnostic Model for Girls with Central Precocious Puberty: Machine-learning Approaches. PloS one. 2022;17(1):e0261965
Figures
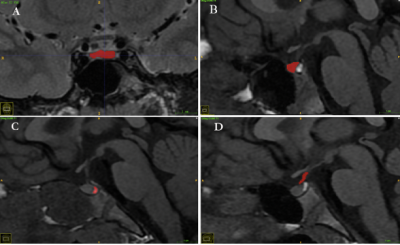
Figure1: Schematic diagram of the segmentation of four regions of interest in pituitary MRI. (A) T2-weighted coronal image anterior lobe. (B) T1-weighted sagittal image anterior lobe. (C) T1-weighted sagittal image posterior lobe. (D) T1-weighted sagittal image Pituitary stalk.
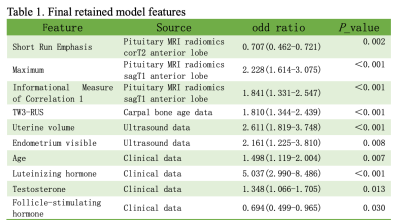
Table 1. Note: Short Run Emphasis in Radiomics is from the Gray-level run-length feature. Maximum is First-order feature. Informational Measure of Correlation 1(LMC1) is from Gray-level co-occurrence Matrix. corT2 = coronal T2-weighted. sagT1 = sagittal T1-weighted
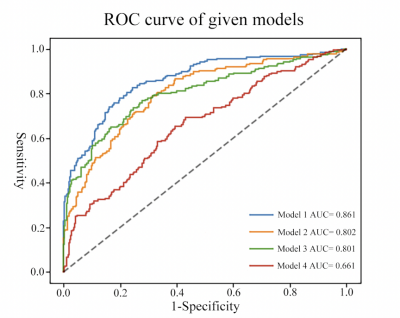
Figure 2: ROC curve of given models. Model 1: Integrated multi-parameter model. Model 2: Basic Clinical model. Model 3: Integrated image model. Model 4: Pituitary MRI radiomics model.