2518
Diagnostic value of adenohypophyseal magnetic resonance imaging features in girls with precocious puberty1Department of Radiology, Tongji Hosptial of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China, 2MR Research, GE Healthcare, Beijing, China
Synopsis
Keywords: Normal development, Endocrine, pituitary gland
It is difficult to distinguish IPP from CPP in the clinical diagnosis. GnRH stimulation test has long been considered the gold standard for the diagnosis of CPP and evaluation of hypothalamic-pituitary-gonadal axis activation, but it is a high cost and an invasive approach. To simplify examination and dig out an alternative method is necessary. In our study, analysis of clinical data, adenohypophyseal MRI features and laboratory characteristics of PP girls help us better comprehensive PP. A combined model of adenohypophyseal MRI features and clinical characteristics improved the diagnostic efficacy of PP and provided us a non-invasive and reliable diagnostic method.Background or Purpose
Precocious puberty (PP) is clinically defined by the development of secondary sexual characteristics (thelarche and pubarche) before the age of 8 years in girls [1]. Central PP (CPP) develops due to premature activation of the hypothalamic-pituitary-gonadal (HPG) axis and is gonadotropin-dependent [2]. Moreover, incomplete PP (IPP) is defined as premature thelarche, premature pubarche, and isolated menarche with incomplete activation of the HPG axis when secondary sexual characteristics occur.IPP is generally the benign variant in puberty, but has the potential to develop into CPP. So, it is important and difficult to distinguish IPP from CPP in clinical diagnosis[3] .The gonadotrophin-releasing hormone (GnRH) stimulation test has been considered the gold standard for the diagnosis of CPP. One drawback of GnRH stimulation test is high cost and invasive operation [4]. The test necessitates hospital admission and multiple samplings. Frequent blood collection can impose a psychological burden on pediatric patients and also cause financial and time-related implications [5].
This study aimed to evaluate the diagnostic value of adenohypophyseal MRI features (adenohypophysis volume [aPV], adenohypophysis height [aPH], and signal intensity ratio [SIR]) for precocious puberty (PP) in girls and also to establish a non-invasive diagnostic approach for easy implement in clinics.
Materials and Methods
A total of 126 girls (37, 57 and 32 girls clinically diagnosed as patients with central PP [CPP] and incomplete PP [IPP], and healthy controls) were enrolled in this study from February 2021 to February 2022. All participants underwent a hypophysial MRI exam and GnRH agonist stimulation tests. Data regarding the adenohypophyseal MRI features and laboratory testing characteristics in the three groups were collected and analyzed using analysis of variance. Pearson correlation analysis was conducted to examine the association. Stepwise multivariate linear regression analysis was used to build prediction models. Receiver operating curve (ROC) analysis was used to evaluate the diagnostic efficacy of MRI features and predicted values.It is difficult to distinguish IPP from CPP in the clinical diagnosis. GnRH stimulation test has long been considered the gold standard for the diagnosis of CPP and evaluation of hypothalamic-pituitary-gonadal axis activation, but it is a high cost and an invasive approach. To simplify examination and dig out an alternative method is necessary. In our study, analysis of clinical data, adenohypophyseal MRI features and laboratory characteristics of PP girls help us better comprehensive PP. A combined model of adenohypophyseal MRI features and clinical characteristics improved the diagnostic efficacy of PP and provided us a non-invasive and reliable diagnostic method.
Results
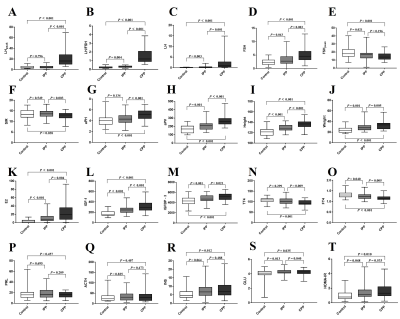
BMI, 17α-OHP, PRL, DHEA‐S, COR, TT3, TSH, TPO and ACTH were not significantly different among the control, CPP, and IPP groups (all P ≥ 0.05). The values of pLH, aPH, TT4 and SIR in the IPP group were not statistically different to control group, but the pLH, aPH, TT4 were respectively significantly lower than the CPP group and the SIR was significantly higher than the CPP group . Both CPP and IPP groups had significantly higher values of basal LH (LH), LHpeak, aPV, Height, Weight, E2, IGF-1, IGFBP-3, GLU and HOMA-IR (all P < 0.05) and lower values of peak FSH (FSHpeak) and FT4 (all P < 0.05) than the control group(Fig.1).Pearson correlation analysis demonstrated that aPV, aPH, Height and Weight were positively associated with LHpeak and LH/FSH (all P < 0.001) while SIR was positively associated with LH/FSH (P = 0.021). The stepwise multivariate linear regression analysis showed predicted LH values (pLH) using aPV, Weight, and aPH as contributors in model 1R2 = 0.271):$$pLH=0.045×aPV+0.484×Weight+1.567×aPH-21.001$$(, and predicted LH/FSH values (pLH/FSH) using SIR, aPV, and Height as contributors in model 2 (R2 = 0.311): $$pLH/FSH=-0.042×SIR+0.002×aPV+0.034×Height-3.686$$ .
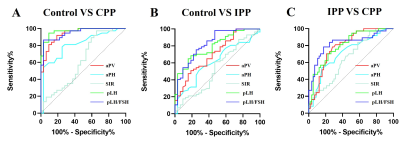
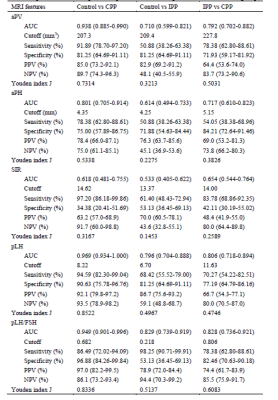
For distinguishing heathy girls from PP, pLH, pLH/FSH and aPV had credible values while for distinguishing different periods of PP, pLH and pLH/FSH had credible values. aPV showed the best diagnostic value among three groups when using adenohypophyseal MRI features alone, but SIR showed the best sensitivity (Fig.2) and table1.
Discussion and conclusions
A model with adenohypophyseal MRI features (SIR) and clinical characteristics (Height and Weight) can rapidly help diagnose IPP, CPP and normal groups with AUC, sensitivity and specificity of 0.828 to 0.949, 78.38% to 86.49%, and 82.46% to 96.88% between each two groups better than the other combined model (aPV/Height/Weight) and only aPV/aPH/SIR.The assessment and management of distinct PP types is important for treatment selection. Inappropriate treatments may lead to ineffectiveness and even worsen children development. With disadvantage of the GnRH stimulation test such as time-consuming, labor-wasting, financial burden, MRI became an alternative approach in evaluation of the pituitary gland and performed in many tertiary care centers to exclude brain abnormalities in CPP-confirmed girls [6]. However, we found aPV was not always precise and accurate in distinguishing IPP from CPP and could only distinguish CPP from the control groups even with AUC, sensitivity and specificity of 0.938, 91.89% and 81.25% at the cutoff value of 207.3 mm3 and other single factors were not, too.
In conclusions, we suggested that diagnosis with our built model (SIR, individual height and weight) can improve and noninvasively obtain a credible diagnostic results in PP types.
Acknowledgements
We thank for Weiyin Vivian Liu (GE Healthcare, Beijing) providing support.References
1. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016 Mar;4(3):265-274. doi: 10.1016/S2213-8587(15)00380-0.
2. Chen M, Eugster EA. Central Precocious Puberty: Update on Diagnosis and Treatment. Paediatr Drugs. 2015 Aug;17(4):273-81. doi: 10.1007/s40272-015-0130-8.
3. Khan SH, Chaudhry N. Beyond GnRH, LH and FSH: The role of kisspeptin on hypothalalmic-pituitary gonadal (HPG) axis pathology and diagnostic consideration. J Pak Med Assoc. 2021 Jul;71(7):1862-1869. doi: 10.47391/JPMA.133.
4. Navarro VM. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol. 2020 Aug;16(8):407-420. doi: 10.1038/s41574-020-0363-7. Epub 2020 May 19.
5.Klein DA, Emerick JE, Sylvester JE, Vogt KS. Disorders of Puberty: An Approach to Diagnosis and Management. Am Fam Physician. 2017 Nov 1;96(9):590-599.
6. Kutlu E, Özgen İT, Bulut H, Koçyiğit A, Otçu H, Cesur Y. Serum Irisin Levels in Central Precocious Puberty and Its Variants. J Clin Endocrinol Metab. 2021 Jan 1;106(1):e247-e254. doi: 10.1210/clinem/dgaa720.
Figures