2517
Effects of Intensive sensorimotor training following experimental cerebral palsy assessed by advanced diffusion MRI1Department of Paediatrics and Gynaecology-Obstetrics, Division of Development and Growth, University of Geneva, Geneva, Switzerland, 2Center for Biomedical Imaging, Animal Imaging Technology section, Federal Institute of Technology of Lausanne, Lausanne, Switzerland, 3Center for Biomedical Imaging, Federal Institute of Technology of Lausanne, Lausanne, Switzerland, 4Institute of Neuroscience, Université catholique de Louvain, Brussels, Belgium, 5Physical and medical rehabilitation department, CHRU Brest, Brest, France, 6Paediatric physical and medical rehabilitation department, Fondation ILDYS, Brest, France
Synopsis
Keywords: Neonatal, Brain, Cerebral palsy, preclinical animal model
Injury to the developing brain is a major cause of Cerebral Palsy (CP) leading to motor and cognitive disabilities. HABIT-ILE is a 2-week intensive sensorimotor rehabilitation program with proven effects decreasing motor impairments in infants with CP. Here, we combined early environmental enrichment (EE) and treadmill motor training (TT) to model HABIT-ILE (EETT) for treating experimental CP in rats assessing then histological and microstructural parameters (diffusion MRI at 9.4T). Exvivo DTI/NODDI showed altered brain microstructure in CP rats not reversed by HABIT-ILE. HABIT-ILE modulated BDNF signaling and decreased the over-expression of proteins involved in excitatory function induced by CP.Introduction
Cerebral palsy (CP) is the most common form of motor disability in childhood, leading to permanent movement disorders, posture, and muscle tone [1]. In rodents, early sensorimotor restriction (from postnatal day P2 to P28) has been shown to cause sensorimotor motor brain maps alterations, H-reflex malfunctioning, increased tonus and musculoskeletal abnormalities and alterations in the gait pattern [2]. HABIT-ILE is a 2-week intensive sensorimotor rehabilitation program with proven effects decreasing motor impairments through plastic changes in white matter tracts in infants with CP. However, the exact mechanisms of this recovery remain undetermined. In this study, we combined early environmental enrichment (EE) and treadmill motor training (TT) to model HABIT-ILE (EETT) for treating CP in rats then assessed brain microstructure changes by advanced diffusion MR imaging at 9.4T and immunoblotting.Materials and Methods
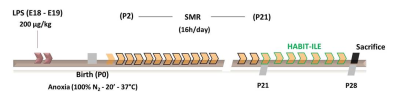
Timeline experiment is summarized in figure 1. Pregnant Wistar rats were divided into 2 groups at embryonic day 18 (E18): CP or Control. CP dams were i.p. injected at E18 and E19 with Lipopolysaccharide (200 μg/kg). On the day of the birth pups from the CP group were submitted to perinatal anoxia (100% N2 during 20 minutes). Following this procedure, from P2 to P21, CP animals were submitted to sensorimotor restriction 16 hours/day using casts made of 1mm diameter metal recovered by hypoallergenic tape. At P21, animals from the CPEETT group (i.e. treated group) were submitted to an experimental HABIT-ILE combining the motor training stimulus (using an automated treadmill designed for rodents - TT) and the sensorimotor experience (using environmentally enriched cages - EE). The intervention lasted from P21 to P27.At P27, rats (8/group, Controls, CP and CPEETT) were sacrificed for subsequent ex-vivo MRI. MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Agilent) equipped with 12-cm gradient coils (400mT/m, 120µs) with a 2.5 mm diameter birdcage coil. A multi-b-value shell protocol was acquired using a spin-echo sequence with the following parameters: FOV(mm2)/matrix size/number of slices/thickness (mm) = 23×18/128×92/20/0.6, axial slices, 3 averages with TE/TR = 45/2000 ms. A total of 96 DWI were acquired, 15 b0 and 81 were separated in 3 shells with the following distribution (# of directions/b-value in s/mm2): 21/1750, 30/3400 and 30/5100 (non-collinear and uniformly distributed in each shell). Diffusivities (Mean, MD; Axial, AD and Radial, RD) and fractional anisotropy (FA) were derived from the tensor by using DTI-TK. Acquired data were fitted using the NODDI toolbox [3] leading to intra-neurite volume fraction (fin), cerebrospinal volume fraction (fiso) and orientation dispersion index (OD). Region of interests (ROIs) were manually delineated in the corpus callosum (CC), cingulate gyrus (CG), basal ganglia (BG), external capsule (EC), motor cortex (MCx) and sensory cortex (SCx). Synaptophysin and BDNF were also quantified by immunoblotting. Statistical tests performed for each technique are explained in the captions of the figures.
Results
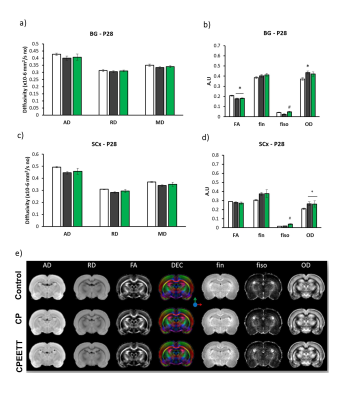
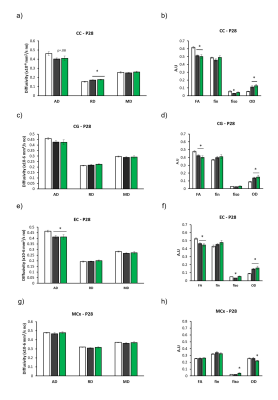
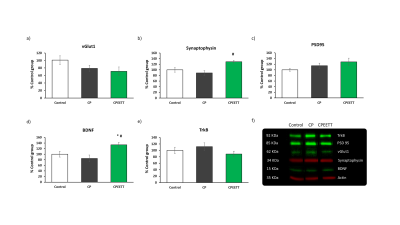
Overall, the model reproduced brain microstructural damages previously reported [4], with increased OD and/or decreased FA in almost all the white matter structures evaluated including CC and EC (Figures 2 and 3). Contrary to our hypothesis, microstructural damages observable with DTI/NODDI were not reversed by HABIT-ILE in most brain regions and partial benefits of HABIT-ILE were observed only for fiso in BG at P28 (F(2,21)=7.27, p<0.01) (Figure 2). In the other hand, Increased GFAP expression in MCx (F(2,18)=5.76, p=0.01), CC (F2,18)=6.29, p<0.05) and hippocampus (F(2.18)=6.52, p<0.05) were reversed by EETT (data not shown). Indeed, In the hippocampus, it was observed an effect of HABIT-ILE increasing expression of synaptophysin (F(2,19)=3.94, p=0.03) and BDNF (F(2,17)=20.14, p<0.01) (Figure 4).Discussion and conclusion
Intensive sensorimotor stimulation such as HABIT-ILE is increasingly considered indispensable in clinical practice in the rehabilitative process. In this study we showed HABIT-ILE-like strategy modulated proteins involved in the cortical excitatory synaptic activity (Synaptophysin), and plasticity (BDNF) in distinct regions of CNS. Despite high quality MR images, we were not able to catch this recovery with diffusion MRI. Further experiments including 1H-MRS and fMRI will be performed to better understand this effect but this study shows that early protocols of physical rehabilitation cause partial neuroprotection following developmental injuries.Acknowledgements
Authors acknowledge Biotech Foundation and Wyss Center for the support with animal experimentation and welfare. This works was supported by the CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, Leenards and Jeantet foundation.References
[1] Andrew MJ, Parr JR, Montague-Johnson C, Braddick O, Laler K, Williams N, et al. Optimising nutrition to improve growth and reduce neurodisabilities in neonates at risk of neurological impairment, and children with suspected or confirmed cerebral palsy. BMC Pediatr 2015;15:22. https://doi.org/10.1186/s12887-015-0339-2.
[2] Clowry GJ, Basuodan R, Chan F. What are the Best Animal Models for Testing Early Intervention in Cerebral Palsy? Front Neurol 2014;5:258. https://doi.org/10.3389/fneur.2014.00258
[3] Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61:1000–16. https://doi.org/https://doi.org/10.1016/j.neuroimage.2012.03.072
[4] Johnston
M v, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and
injury in the developing brain. Brain and Development 2009;31:1–10.
https://doi.org/https://doi.org/10.1016/j.braindev.2008.03.014.
Figures