2515
Cortical alterations after very preterm birth and the association with socio-emotional abilities from childhood to early adolescence1Computer Imaging and Machine Learning, CIBM CHUV-EPFL SP Section, Lausanne, Switzerland, 2Division of Development and Growth, Department of Paediatrics, Gynaecology and Obstetrics, Geneva University Hospitals, Geneva, Switzerland, Geneva, Switzerland, 3Institute of Bioengineering, Center for Neuroprosthetics, Ecole Polytechnique Fédérale de Lausanne, Switzerland, Lausanne, Switzerland, 4Department of Radiology and Medical Informatics, Faculty of Medicine, University of Geneva, Switzerland, Geneva, Switzerland, 5SensoriMotor, Affective and Social Development Laboratory, Faculty of Psychology and Educational Sciences, University of Geneva, Geneva, Switzerland, Geneva, Switzerland, 6Division of Development and Growth, Department of Paediatrics, Gynaecology and Obstetrics, Geneva University Hospitals, Geneva, Switzerland
Synopsis
Keywords: Adolescents, Pediatric
This study compared GM concentration and its developmental trajectory in vey preterm (VPT) and full-term (FT) children aged 6 to 14 years. Widespread abnormal GM concentration was found with complicated patterns of increases/decreases of GM concentration across cortical and subcortical regions. Socio-emotional abilities were association with GM concentration in regions known to be involved in such process for both VPTand FT peers. Our findings suggest the trajectory of brain development following VPT birth may be fundamentally distinctive with impact on socio-emotional abilities.Introduction
For very preterm infants (VPT < 32 weeks' gestation) crucial steps of brain development occur in an abnormal ex-utero environment, leading to altered cortical and subcortical development. Associated with this atypical brain maturation, VPT are at high risk of socio-emotional difficulties later in life. The study of GM alteration in individuals born preterm have traditionally been conducted using volumetric measurements based on the binary classification of signal intensities of the T1-weighted image1–5. However, the complexity of GM properties might not be fully capture using such volumetric measures. During early brain development, the cortical plate is composed of GM and WM. The cortex is populated by neurons that migrate on glial cells radially towards the pial surface (the boundary between GM and cerebrospinal fluid (CSF))6. Following this migration, cross-connections develop, with dendrites and axons obscuring this radial structure7. Hence, the tissue architecture of cortical GM is made by a characteristic organization of neurons, glia cells and more or less myelinated axons. The different tissue characteristics in a single voxel makes the estimation of GM-WM volumes based on signal intensities challenging. By describing the intensity within each voxel as the sum of GM, WM and CSF characteristic intensities one can estimate the signal intensities of several tissue types in a single voxel, in contrast of a binary classification based on global signal averaging.Methods
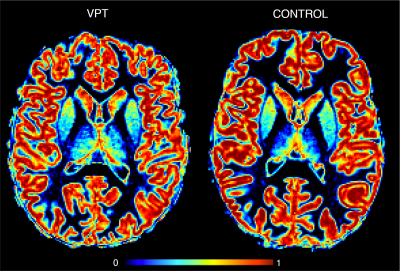
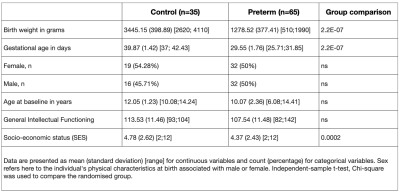
Participants were recruited in the context of two intervention studies (‘Mindful preterm teens’ study 8 and ‘Vis-à-Vis’ study), at the age of 6 to 14 years, between January2017- July2019 (demogprahics in table1).High-resolution T1-weighted images (0.9mm isotropic) were acquired: MPRAGE; TE=2.3s; 256x256, 192 sagittal slice (3T Siemens Prisma, Erlangen, Germany). T1 images were used to extract GM concentration by taking into account partial volume effect as in9 (Fig 1). Socio-emotional abilities were assessed at the time of the MRI scan in all participants using neuropsychological testing as well as parent-reported questionnaires specifically testing socio-emotional abilities10. Group-wise differences in cortical GM concentration between the VPT and FT groups were evaluated using a general linear model (GLM) as implemented in Freesurfer.
The ROI-based averaged GM concentration was used to evaluate the multivariate patterns of correlation between the GM concentration measures and socio-emotional scores, using a multivariate partial least square correlation (PLSC), instead of a mass univariate approach. We used an openly available Matlab code11, running in MATLAB vR2019b version (The MathWorks, Inc., Natick, MA).
Results
No significant difference was found in terms of general intellectual functioning between the VPT and FT. FT participants showed a significantly higher SES compared to VPT (p<.005, table1). Compared to FT, VPT showed atypical GM concentration in a range of brain regions that seem to not simply result in decreases and increases in GM concentration, but in complicated patterns of alterations (Fig2).Compared to FT, VPT showed atypical GM concentration in a range of brain regions that seem to not simply result in decreases and increases in GM concentration, but in complicated patterns of alterations (Fig 2).
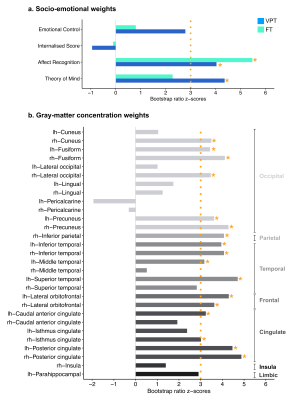
Overall, better socio-emotional abilities were associated with increased GM concentration for both VPT and FT. The PLSC analysis gave one significant latent component (LC1, p=0.016). LC1 revealed comparable patterns of associations in the VPT and full-term control group with better socio-emotional abilities associated with increased GM concentration. Both in the VPT and full-term control group, results show an association between increased Affect Recognition scores with increased GM concentration means bilaterally in the FFG, PCUN, ITG, LOFC and PCC; in the left cACC, MTG and STG; as well as in the right CUN, ICC, LOCC and IPC. In the VPT group, this same pattern of increased GM concentration means in the same ROIs was also associated with increased Theory of Mind scores (Fig 3)
Discussion
Overall, our results demonstrated that VPT birth is associated with extensive alterations in cortical and subcortical brain structure in children and adolescence, that seems particularly widespread in occipital and temporal regions. As illustrated above and already reported in the literature, the complex pattern of both increases and decreases in cortical and subcortical GM concentration, GM volume, thickness and surface areas in the different regions compared to full-term controls defy an easy generalization with respect to their developmental significance. As described by Nosarti et al.12 this complex pattern of GM distribution can be interpreted within a "neuroplastic" framework, which proposed that developmental changes in any brain region may result in a cascade of alterations in many other regions. Nevertheless, this study adds to the evidence that perinatal complications, such as VPT birth, that occur at critical periods of development disrupt maturation and have a long lasting effect on subsequent brain development13,14. These findings fail to find evidence for a developmental “catch-up” in VPT children and adolescents that was previously suggested in the literature15. One possibility is that this “catch up” occurs later in development, during the late adolescence. However, these findings are also in line with other studies exploring GM developmental trajectories16,17, suggesting the trajectory of brain development following VPT birth may not only be delayed, but also fundamentally distinctive.Acknowledgements
We thank and acknowledge all participating children and adolescents as well as their families who made this research possible. We also thank the Fondation Campus Biotech Geneva (FCBG), a foundation of the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), and the University Hospitals of Geneva (HUG). Finally, we extend our gratitude to the paediatric clinical research Platform of the Geneva University Hospital for their help in data management.References
1. Ment, L. R. et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics123, 503–511 (2009).
2. Kesler, S. R. et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J. Pediatr. 152, (2008).
3. Kesler, S. R. et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr. Neurol. 31, 318–325 (2004).
4. Soria-Pastor, S. et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics 124, (2009).
5. Botellero, V. L. et al. A longitudinal study of associations between psychiatric symptoms and disorders and cerebral gray matter volumes in adolescents born very preterm. BMC Pediatr. 17, (2017).
6. Bystron, I., Blakemore, C. & Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 9, 110–122 (2008).
7. Vasung, L. et al. Quantitative and Qualitative Analysis of Transient Fetal Compartments during Prenatal Human Brain Development. Front. Neuroanat. 10, (2016).
8. Siffredi, V. et al. The effect of a mindfulness-based intervention on executive, behavioural and socio-emotional competencies in very preterm young adolescents. Sci. Rep. 11, 1–12 (2021)
9. Fischi‐Gomez, E., Bonnier, G., Ward, N., Granziera, C. & Hadjikhani, N. Ultra‐high field in vivo characterization of microstructural abnormalities in the orbitofrontal cortex and amygdala in autism. Eur. J. Neurosci. 1–8 (2021) doi:10.1111/ejn.15420.
10. Siffredi, V. et al. Large-scale brain network dynamics in very preterm children and relationship with socio-emotional outcomes: an exploratory study. Pediatr. Res. 2022 1–9 (2022) doi:10.1038/s41390-022-02342-y.
11. Zöller, D. et al. Psychotic symptoms influence the development of anterior cingulate BOLD variability in 22q11.2 deletion syndrome. Schizophr. Res. 193, 319–328 (2018).
12. Nosarti, C. et al. Preterm birth and psychiatric disorders in young adult life. Arch. Gen. Psychiatry 69, (2012).
13. Petanjek, Z. & Kostović, I. Epigenetic regulation of fetal brain development and neurocognitive outcome. Proc. Natl. Acad. Sci. 109, 11062–11063 (2012).
14. Raznahan, A., Greenstein, D., Lee, N. R., Clasen, L. S. & Giedd, J. N. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc. Natl. Acad. Sci. U. S. A. 109, 11366–11371 (2012).
15. Nam, K. W. et al. Alterations in cortical thickness development in preterm-born individuals: Implications for high-order cognitive functions. Neuroimage 115, 64 (2015).
16. Karolis, V. R. et al. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. Neuroimage 163, 379–389 (2017).
17. Gale-Grant, O. et al. Effects of gestational age at birth on perinatal structural brain development in healthy term-born babies. Hum. Brain Mapp. 1–13, 2021.05.02.442327 (2021).
Figures