2511
Deep learning-based DWI connectome analysis to improve the prediction of postoperative language improvement in pediatric epilepsy
Min-Hee Lee1,2, Nathan Sim3, Marie Papamarcos3, Masaki Sonoda4, Csaba Juhász1,2, Eishi Asano1,5, and Jeong-Won Jeong1,2
1Pediatrics, Wayne State University, Detroit, MI, United States, 2the Translational Imaging Laboratory, Children's Hospital of Michigan, Detroit, MI, United States, 3Medical Doctor Program, Wayne State University, Detroit, MI, United States, 4Neurosurgery, Yokohama City University, Yokohama, Japan, 5Neurology, Children's Hospital of Michigan, Detroit, MI, United States
1Pediatrics, Wayne State University, Detroit, MI, United States, 2the Translational Imaging Laboratory, Children's Hospital of Michigan, Detroit, MI, United States, 3Medical Doctor Program, Wayne State University, Detroit, MI, United States, 4Neurosurgery, Yokohama City University, Yokohama, Japan, 5Neurology, Children's Hospital of Michigan, Detroit, MI, United States
Synopsis
Keywords: Neuro, Epilepsy, Prediction of postoperative language improvement in children with epilepsy
We present a novel deep learning-based tract classification to effectively remove false positive tract streamlines from preoperative DWI connectome data of children with medically intractable epilepsy. Compared to the prediction model without the presented classification where uncontrollable false positive tracts significantly limit the accurate prediction of postoperative language improvement using local efficiency values of key hub regions in the receptive and expressive language networks, the prediction model with the presented classification enhanced the accuracy of about 34% up to 100%/88% for the prediction of receptive/expressive language improvement, especially when the local efficiency values were combined with the clinical variables.Introduction
Postoperative language improvement is known as one of the potential benefits from pediatric epilepsy surgery1. To extract new imaging predictors associated with child language development, DWI connectome (DWIC) has been actively used in recent studies2,3. However, its inter-subject variability highly depends on the uncontrollable content of false positive tract streamlines4 that significantly limit statistical inference of clinically acquired DWIC. This study proposes a novel deep convolutional neural network (DCNN)5 to automatically remove the false positive streamlines by objectively predicting highly reproducible true positive streamlines (i.e., those consistently present across subjects) via an end-to-end deep learning of reference streamline coordinates defined in high quality DWI data: the human connectome project (HCP). Using the proposed method, we evaluated the effectiveness of a novel deep learning-based DWIC analysis that may remarkably improve the prediction of postoperative language outcome using clinically acquired preoperative DWIC data.Methods
HCP 3T DWI data of a pediatric cohort (https://db.humanconnectome.org/) were used to define reference streamlines in the whole-brain that we targeted to reproduce in clinical DWIC. Briefly, the MRtrix package (http://www.mrtrix.org/) was used to generate a group template of fiber orientation distribution (FOD)6. Using this template, 50 million tracts were generated by applying SIFT1 reconstruction to 100 million iFOD2-ACT whole-brain tracts7. The AAL parcellation atlas (http://www.gin.cnrs.fr/en/tools/aal-aal2/) was then used to create a whole-brain backbone DWIC, S{k,l}, in which each element defines a reference streamline class, Ci=1,2,…,M, consisting of a series of streamlines: fj connecting both kth and lth regions (i.e., M = 1477 is total number of Ci existing in the whole-brain backbone network S{k,l}). For each of Ci=1,2,...,1477, two raters (M.L. and N.S.) manually cleaned up all visible false positive streamlines (i.e., wiggly fibers and broken fibers) to define the ground-truth streamlines fj. 70% and 30% of the resulting round-truth streamlines were used to construct training and test sets, respectively. For each fj of the training Ci, our DCNN model was designed to learn 3-D (x,y,z) coordinates of 100 equal-number streamline segments by minimizing center loss5. After training, the fully connected layer produced the output probability vector, P(Ci=1,2,…,1477|fj): the prediction probability of the input fj belonging to the class Ci. An argument of maximum in P(Ci=1,2,…,1477|fj) was used to predict a class membership of the input fj. To demonstrate the efficacy of the proposed DCNN to predict postoperative language improvement using preoperative DWIC, 18 children with medically intractable epilepsy (age:13.0±3.4 years) underwent pre- and postoperative DWI at a 3T GE scanner using 55 encoding directions at b=1000 s/mm2 (average scan interval = 1.6 years) and also received pre- and postoperative neuropsychological assessments of expressive and receptive language skills using the clinical evaluation of language fundamental (CELF) test8 (average evaluation interval = 2.5 years revealing that 7 and 4 patients improved expressive and receptive language skills after surgery, respectively). The same connectome procedure used for the HCP data was applied to individual DWI data in order to generate the DCNN-based DWIC S{k,l} in the HCP template space. Local efficiency (LE, the inverse of the average shortest path connecting all neighbor nodes) values of key hub regions in each of the expressive and receptive modular language networks2 were extracted from the DCNN-based DWIC and the original backbone DWIC, respectively. The presence of postoperative language improvement was predicted using a binary regression model with the extracted LE values and 3-fold cross-validation.Results
We found that DCNN improved the inter-subject intra-class correlation of the backbone DWIC S{k,l} (R = 0.91/0.93 without/with DCNN), suggesting that our DCNN helps improve inter-subject reproducibility of the backbone DWIC S{k,l} by effectively removing noise tracts and false-positive tracts (Fig. 1). This improvement directly led to an enhanced prediction of postoperative language improvement using the LE values of key hub regions in both the receptive and expressive language networks (34% improvement of AUC on average, Fig. 2). Finally, the LE values combined with the clinical variables yielded the outstanding prediction of postoperative receptive language improvement using the preoperative DWIC (AUC = 1.00/0.88 for receptive and expressive language, respectively, Fig. 3).Discussion
The present study provides preliminary evidence that a novel DCNN-based DWIC analysis can identify patients with a potential to benefit with postoperative language improvement. Pediatric epilepsy patients often undergo low angular resolution scans for DWI tractography to limit MRI scan time. This sparse encoding scheme inevitably produces uncontrollable false-positive tracts and increases the inter-subject variability of clinical DWIC data, significantly limiting its prediction accuracy. Our analysis overcomes this limitation by translating the advanced DCNN-based tract classification to clinical DWIC with sparse encoding. Further investigation of this approach should evaluate if it helps improve the reproducibility of clinical DWIC across multi-institutional data.Conclusion
Our findings suggest a promise of our DCNN-based tract classification to predict postoperative language improvement more accurately via intelligently removing false positive tract streamlines without significantly depending on a sparse DWI encoding scheme.Acknowledgements
This research was supported by grants from the National Institutes of Health, R01 NS089659to J.J. and R01 NS064033 to E.A.References
- Kaur N, Nowacki AS, Haut JS, et al. Cognitive outcomes following pediatric epilepsy surgery. Epilepsy Res 2022 Feb;180:106859.
- Lee MH, O'Hara NB, Behen ME, et al. Altered efficiency of white matter connections for language function in children with language disorder. Brain Lang. 2020;203:104743.
- Jeong JW, Lee MH, O'Hara N, et al. Prediction of baseline expressive and receptive language function in children with focal epilepsy using diffusion tractography-based deep learning network. Epilepsy Behav. 2021;117:107909.
- Prčkovska V, Rodrigues P, Puigdellivol Sanchez A, et al. Reproducibility of the structural connectome reconstruction across diffusion methods. J Neuroimaging. 2016;26(1):46-57.
- Xu H, Dong M, Lee MH, et al. Objective detection of eloquent axonal pathways to minimize postoperative deficits in pediatric epilepsy surgery using diffusion tractography and convolutional neural networks. IEEE Trans Med Imaging. 2019;38(8):1910-1922.
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007; 35(4):1459–1472.
- Smith RE, Tournier JD, Calamante F, et al. SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013;67:298-312.
- Paslawski T. The clinical evaluation of language fundamentals, fourth edition (CELF-4): A Review. Can J Sch Psychol. 2005;20:129-134.
Figures
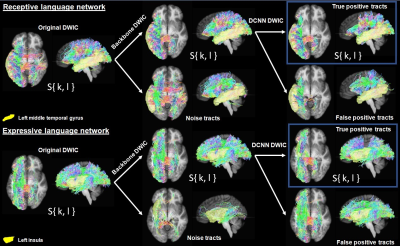
Figure
1. The proposed DCNN
classification applied to original DWIC S{k,l} to construct
DCNN-based DWIC S{k,l} that is composed of true positive tracts for the receptive
language network (top box, {fn(x,y,z)} of Ci connecting the left middle temporal gyrus to
the other hub regions of receptive language network) and expressive language
network (bottom box, {fn(x,y,z)} of Ci connecting the left insular to the other hub
regions of expressive language network).
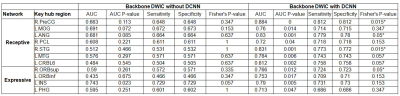
Figure 2. Prediction of postoperative language
improvement using local efficiency values of key hub regions in receptive and
expressive language networks that were obtained from preoperative backbone DWIC
S{k,l} without DCNN tract classification (left) and with DCNN tract classification
(right). AUC stands for the area under curve obtained from the binary logistic
regression model. An asterisk indicates a statistically significant prediction
in the 2x2 contingency table relative to the LE value of the given key hub
region. The atlas labels are available in our previous work2.
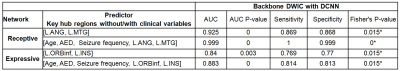
Figure 3. Prediction of postoperative language
improvement using local efficiency values of two key hub regions of the
preoperative backbone DWIC S{k,l} with DCNN tract classification (i.e., L.ANG
and L.MTG for receptive language, L.ORBinf and L.INS for expressive language)
and three clinical variables measured before surgery (i.e., age, total number
of antiepileptic drugs tried before surgery [AED], seizure frequency before
surgery). Receptive and expressive language networks were obtained from the
preoperative backbone DWIC S{k,l} with DCNN tract classification (right).
DOI: https://doi.org/10.58530/2023/2511