2506
Amide Proton Transfer (APT) Imaging in pediatric low grade glioma versus non neoplastic intracranial lesion1IRCCS Istituto Giannina Gaslini, Genova, Italy, Genova, Italy
Synopsis
Keywords: Tumors, Brain
Many non-neoplastic pediatric neurological diseases could often mimic brain tumors in conventional neuroimaging. Amide proton transfer-weighted (APTw) imaging adds important value to standard MR imaging sequences in brain tumor diagnosis. The high APT signal of neoplastic lesions seems to be related to the high cellularity of brain tumors and to overexpression of many proteins compared to normal brain tissue. We demonstrate that non-neoplastic lesions are characterized by APT values significantly lower than low grade gliomas (LGGs). APTw imaging may lead to an increased accuracy in differentiating LGGs from non-neoplastic lesions in pediatric patients.
Introduction
Magnetic resonance (MR) imaging is the most important imaging modality for the diagnosis of pediatric central nervous system (CNS) diseases. It is sometimes difficult to effectively distinguish pediatric non-neoplastic from neoplastic brain lesions using conventional MRI. Conventional MRI features of many intracranial non-neoplastic diseases, such us demyelinating lesion, focal cortical dysplasia (FCD), hypothalamic amartoma, infectious disease or granulomatous lesions can mimic low-grade glioma. However, the treatment strategies and prognosis of these two pathological entities are completely different, therefore it is very important to differentiate them. Amide proton transfer-weighted (APTw) imaging is a chemical exchange saturation transfer (CEST)-based molecular MR imaging technique that is sensitive to endogenous mobile proteins and peptides in tissue, such as those dissolved in the cytoplasm 1. Furthermore, APTw imaging is based on endogenous contrast agents, so no exogenous contrast agent injection is required, particular benefits for pediatric patients 1. Early data have demonstrated that APTw imaging adds important value to standard MR imaging sequences in the clinical setting. Few previous studies have analyzed the value of this sequence in assessing intracranial infectious lesions and in differentiating high from low-grade intracranial tumors as well as neoplasia from infection 1-3. In this study, we investigated the feasibility of APT imaging for differentiating low-grade gliomas and non neoplastic brain lesions in pediatric patients.Methods
A total of 35 patients from our institution were selected for this retrospective study, of which 20 with confirmed LGG and 15 presenting a non-neoplastic lesion (no-LGG) with available APT images at onset or previous to treatment/surgery. MRI acquisition was preformed in our centre on a 3T scan using a 32 channel head array coil (Igenia Cx, Philips, Best, the Netherlands). A semi-automated segmentation approach was used to discriminate the lesion from the normal tissue. Two volumes of interest (VOI), the whole lesion and the contralateral normal appearing brain (CLNAB) tissue. Registration of the images, the semi-automated segmentation process and histogram metrics calculation were achieved using the Philips Itellispace portal for Windows (v. 8).Mean APTw and normalised (nAPTw), described as the difference between the lesion and the CLNAB mean, were measured and compared between the LGG group and the no – LGG group.The non-parametric test Mann U Whitney was used to compare the medians of the two groups. A receiver operating characteristics (ROC) curve analysis was performed. Statistical analysis was computed through the SPSSstatistic package for Mac (v. 21.0.0.0) and Matlab (v. R2019a, Mathworks).Results
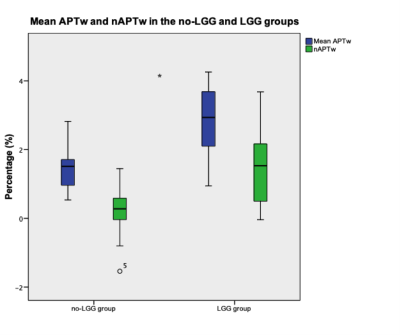
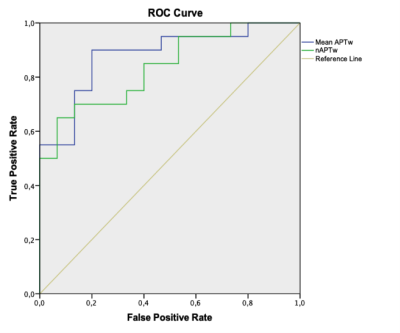
The analysis on the mean APTw and nAPTw revealed lower values for the no-LGG group compared to the LGG group (Graph 1.). Specifically, for the no-LGG group, the mean APTw and nAPTw were 1.49 ± 0.64 % and 0.21 ± 0.53 %, respectively; whereas LGG group means were 2.86 ± 0.73 % and 1.52± 1.08 %. A p-value of < 0.001 was estimated.The ROC curve was preformed for APTw and nAPTw (Graph 2.). An AUC of > 0.837 was calculated for both metrics (SE<0.06, p-value < 0.001). We found that an optimal cut-off value for the APTw was 1.725 (TPR= 0.90 and FPR= 0.20) and 0.5045 (TPR =0.75 and FPR = 0.33) for the nATPw.Discussion
In the present study, we evaluated the utility of ATP in differentiating LLG and non-neoplastic lesions. Many non-neoplastic pediatric neurological diseases could often mimic brain tumors in neuroimaging and pose a diagnostic dilemma which sometimes may result in unnecessary surgery. In some cases, such as in cortical dysplasia, LGG and dysplastic lesions can coexist. Numerous studies 4-7 indicate that APTw imaging may add important value to brain tumor diagnosis. The high APT signal of neoplastic lesions seems to be related to the high cellularity of brain tumors and to overexpression of many proteins compared to normal brain tissue 8. Another possible contribution to increased protein signal in malignant tumors is angiogenesis, as the blood contains high concentrations of hemoglobin and albumin 9. Our study, according to previous studies, demonstrates that no-LGG group is characterized by APT values significantly lower than LGG group.Conclusion
APTw imaging may lead to an increased accuracy in differentiating LGGs from non neoplastic lesions.Acknowledgements
No acknowledgement found.References
1. Zhang, H., Zhou, J., & Peng, Y. (2021). Amide Proton Transfer–Weighted MR Imaging of Pediatric Central Nervous System Diseases. Magnetic Resonance Imaging Clinics, 29(4), 631-641.
2. Zhang, H., Yong, X., Ma, X., Zhao, J., Shen, Z., Chen, X., ... & Zhang, Y. (2021). Differentiation of low-and high-grade pediatric gliomas with amide proton transfer imaging: added value beyond quantitative relaxation times. European Radiology, 31(12), 9110-9119.
3. Zhang, H., Tang, X., Lv, Y., Hu, D., Sun, J., Wang, Y., ... & Peng, Y. (2020). Amide proton transfer-weighted (APTw) imaging of intracranial infection in children: initial experience and comparison with gadolinium-enhanced T1-weighted imaging. BioMed research international, 2020.
4. Wen, Z., Hu, S., Huang, F., Wang, X., Guo, L., Quan, X., ... & Zhou, J. (2010). MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage, 51(2), 616-622.
5. Jiang, S., Yu, H., Wang, X., Lu, S., Li, Y., Feng, L., ... & Wen, Z. (2016). Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. European radiology, 26(1), 64-71.
6. Yu, H., Lou, H., Zou, T., Wang, X., Jiang, S., Huang, Z., ... & Wen, Z. (2017). Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. European radiology, 27(11), 4516-4524.
7. Sakata, A., Fushimi, Y., Okada, T., Arakawa, Y., Kunieda, T., Minamiguchi, S., ... & Togashi, K. (2017). Diagnostic performance between contrast enhancement, proton MR spectroscopy, and amide proton transfer imaging in patients with brain tumors. Journal of Magnetic Resonance Imaging, 46(3), 732-739.
8. Van Zijl, P. C., Zhou, J., Mori, N., Payen, J. F., Wilson, D., & Mori, S. (2003). Mechanism of magnetization transfer during on‐resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 49(3), 440-449.
9. Zhou, J., Heo, H. Y., Knutsson, L., van Zijl, P. C., & Jiang, S. (2019). APT‐weighted MRI: Techniques, current neuro applications, and challenging issues. Journal of Magnetic Resonance Imaging, 50(2), 347-364.