2482
Quantitative phase contrast MRI of penetrating arterioles in cerebral white matter in patients with diabetes at 7T
Xiaopeng Zong1, Jordan Jimenez2, Tengfei Li2, and William Powers3
1ShanghaiTech University, Shanghai, China, 2University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Duke University, Durham, NC, United States
1ShanghaiTech University, Shanghai, China, 2University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Duke University, Durham, NC, United States
Synopsis
Keywords: Blood vessels, Aging, small vessel disease
Brain lesions caused by cerebral small vessel disease (SVD) are commonly observed in the elderly. However, it remains challenging to noninvasively measure the early pathological changes of the underlying vessels. To this end, we evaluated the feasibility of detecting changes in white matter penetrating arterioles (PA) with 7T MRI in patients with diabetes, a known risk factor for SVD, but without severe SVD and in age and gender matched healthy controls (HC). We observed lower flow velocities in PAs in the patients, suggesting that early changes in PA that are discriminative of overt SVD risks can be detected at 7T.INTRODUCTION
Cerebral small vessel disease is responsible for primary intracerebral hemorrhages, lacunar infarcts and leukoaraiosis. It is associated with substantial cognitive, psychiatric and physical disabilities. While the brain lesions attributed to small vessel disease can be characterized by conventional MRI due to their larger sizes and clear visualization, it remains challenging to noninvasively measure the early pathological changes of the small underlying vessels. An imaging method for measuring the early structural and functional changes of cerebral small vessels in vivo would illuminate the etiopathogenesis of cerebral small vessel disease and help to develop effective treatment strategies. To this end, we evaluated the feasibility of detecting changes in white matter penetrating arterioles (PA) with ultra-high field 7T MRI in patients with diabetes but without severe SVD.METHODS
19 participants with diabetes mellitus (DM) and 19 age- and gender-matched healthy controls were recruited. The Mini MoCA version 2.1 was administered to each participant to evaluate different cognitive domains, including attention, concentration, executive functions, memory, language, and orientation. Blood glucose level was measured using a Care Touch Diabetes Testing Kit (Brooklyn, NY, USA) prior to the MRI scan in each subject. Additional clinical data including medical history and cardiovascular risk factors were collected through a questionnaire or retrieved from medical records when available. Lipid panels and A1C levels are reported only if they were measured within 180 days prior to the MRI scan. All images were acquired using a Nova 32-channel receiver and single-channel volume transmitter coil on a 7T MRI scanner. To evaluate possible presence of typical SVD features including white matter hyperintensities (WMH), lacunar infarct, and microbleed, high resolution whole-brain turbo spin echo and susceptibility weighted imaging scans were performed. To image the PAs, single slice phase contrast (PC) MRI covering the centrum semiovale was acquired. The PC-MRI scan had a VENC of 4 cm/s with one-sided encoding. The slice was positioned 15 mm above the corpus callosum and parallel to the anterior commissure – posterior commissure line. Prospective motion correction was performed during the TSE and PC-MRI scans based on fat navigator images acquired throughout the scans.[1] PA masks were manually drawn by one of the authors (XZ) on the PC-MRI image from each subject, after anonymizing the images. For each manually drawn PA, lumen diameter (DPA), flow velocity (VPA) and volume flow rate (QPA) were derived by model based analyses of complex difference image (MBAC), which was necessary for correcting the strong partial volume effects in the PA signal.[2] Quasi-Poisson regression was performed for PA count using disease condition, age, and gender as independent variables because the residual deviance from Poisson regression showed significant overdispersion (dispersion test, p=6.8×10-6). Linear mixed effect model analyses were performed for the other measurements using disease condition as the single fixed effect and a random effects term representing the pairs of participants and another random effect term representing the participants within the pairs.RESULTS
DM participants had higher weight, body mass index, systolic blood pressure, blood glucose level, and more with hypertension and a history of smoking. MoCA cognitive scores, lipid panel results, and diastolic blood pressure were comparable between groups. No microbleeds were found in any participant and only one DM participant had a 5 mm lacunar infarct in the caudate nucleus. WHM was observed in 14 and 6 DM and control subjects, respectively, but none of the subjects had Fazekas score above 2. PA diameters ranged from 80 to 500 microns as shown in Figure 1. Flow velocities were lower in the DM group (1.9 ± 0.63 vs. 2.2 ± 0.63 cm/sec, p=0.02). The distributions of velocity are shown in Figure 2. None of the p-values for between group differences of PA count, DPA and QPA were < 0.05.DISCUSSION
This study demonstrates that sizes and flows of individual PAs discriminative of disease state can be quantitatively measured in human cerebral WM with 7T MRI in vivo. The observed decrease in PA flow velocity in DM participants is consistent with increased tortuosity. Increased PA tortuosity can reduce flow induced phase changes because the flow directions can deviate more from the direction of velocity encoding gradient in tortuous PA leading to a decrease in measured velocity.[3] Furthermore, in the absence of changes in vessel diameter that alter volume flow, velocity is proportional to the square of the radius and thus can be a more sensitive indicator than diameter itself of early vessel changes in size.CONCLUSIONS
This study demonstrates that sizes and flows of individual PAs that are discriminative of disease state can be quantitatively measured in human cerebral white matter with 7T MRI in vivo. These results provide a basis for further studies to characterize the alterations in white matter PA and their role in producing the overt clinical and imaging manifestations of cerebral small vessel disease.Acknowledgements
The project described was partly supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. We thank Dr. John Buse at the Department of Endocrinology, the University of North Carolina at Chapel Hill for suggestions, and Dr. Andrea Bozoki at the Department of Neurology, University of North Carolina at Chapel Hill for recommending the MoCA test.References
1. Moore J, Jimenez J, Lin W, Powers W, Zong X. Prospective motion correction and automatic segmentation of penetrating arteries in phase contrast mri at 7 t. Magn Reson Med. 2022 2. Zong X, Lin W. Quantitative phase contrast mri of penetrating arteries in centrum semiovale at 7t. NeuroImage. 2019;195:463-474 3. Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203-204:159-163Figures
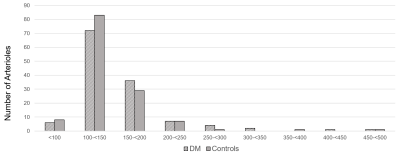
Figure 1: Histogram of the diameters of penetrating arterioles in 129 vessels from 19 participants
with diabetes mellitus (DM) and 130 vessels from 19 controls.
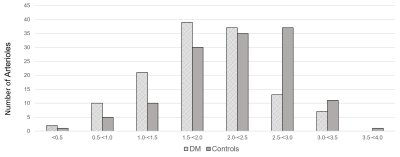
Figure 2: Histogram of the velocities of penetrating arterioles in 129 vessels from 19 participants
with diabetes mellitus (DM) and 130 vessels from 19 controls.
DOI: https://doi.org/10.58530/2023/2482