2480
Pulsatility in the circle of Willis increases with age1Cornell University, Ithaca, NY, United States, 2Weill Cornell Medicine, New York, NY, United States
Synopsis
Keywords: Blood vessels, Velocity & Flow, Pulsatility, Pulse waves
As we get older, the elasticity of our blood vessels decreases significantly, which can lead to a diminished absorption of blood pressure pulsations, potentially causing damage including hemorrhagic stroke and microbleeds. We studied pulsatility in the circle of Willis, which has been hypothesized to be a pressure absorber, by the method of MRI hypersampling by analytic phase projection (APP). A significant correlation of pulsatility in the circle of Willis with age was found. This finding is interpreted as an ageing-related increase in pulsatile flow relative to the steady flow component of overall cerebral blood flow.Introduction
Cerebrovascular pulsatility has become an important research topic due to its possible role in ageing-related brain health. As we get older, the viscoelasticity of our blood vessels decreases significantly1-5, which can lead to diminished absorption of blood pressure pulsations, potentially causing damage including hemorrhagic stroke and microbleeds. Ageing-related changes of the central and peripheral cardiovascular system affect blood-flow, pulsatility, and pathologies of the brain, too6-9. In addition, the arterial pulsatile pressure gradient could be a driving force in the brain’s paravascular waste clearance system.10,11The region under study is the circle of Willis (COW), which has been hypothesized as a pressure absorber mechanism that prevents damage of the cranial microvasculature and blood brain barrier12. Recent progress has been achieved in imaging pulsatility13-23 and pulse waves24-28 in the brain with fast echo-planar imaging (EPI). We use the method of MRI hypersampling by analytic phase projection (APP)25,27,28 to study the very fast pulse waves, which are significantly faster than blood flow velocity. APP overcomes the arbitrary limitation of retrospective gating that the phase within an inter-beat interval is assumed to increase linearly; rather, the phase is estimated from the analytic phase of the pulse oximetry signal via a Hilbert transform.
Methods
Subjects: Out of a total of 38 healthy subjects, after quality control 28 participants between 20 to 72 years of age (17 female) were included in the analysis. The young group included 16 subjects (20 ~ 28 years, and 12 female) and the older group 12 subjects (61 ~ 72 years, 5 female). The study was approved by the Institutional Review Board of Columbia University Irving Medical Center.Imaging: Subjects were imaged using a 3 Tesla Siemens Magnetom Prisma MRI an ultrafast echo-planar imaging (EPI) sequence with TR =100 ms. One slice passing through the COW was imaged with 3000 repetitions totaling 5 min scan time.
Pulse waveform analysis: The pulse oximetry signals were filtered to ensure pseudo-periodicity29-31 of the pulse signal, and to remove noise. MRI pulse waveforms were extracted from the motion-corrected, detrended EPI data by APP. The pulse oximetry signals were shifted in time to match the MRI acquisition sample times. Pulse waveforms were estimated by smoothing the hypersampled MRI signal. The effective hypersampling time was approximately 0.3 ms.
Statistics: Two circular regions of interest (ROIs) were defined for each fMRI data set, one in the COW and one for the baseline (Figure 1, left). The MRI pulse amplitude was computed as the average of the differences between the maximum and minimum waveform value inside the ROI. In the COW, this amplitude was then divided by the median amplitude from the baseline ROI. A multiple regression analysis was performed with MRI pulse amplitudes in the COW as dependent variable and age, heart rate, motion, and gender as independent variables.
Results
Pulse amplitude maps showed pulsatility in the circle of Willis, parts of the middle cerebral arteries, arteries in the scalp, and other regions (Figure 1). The pulse amplitude in the COW increased with age (p = 0.012; Figure 2) but was not significantly dependent on heart rate, gender, or head motion during MRI. In the baseline region, pulse amplitude did not depend on any of the covariates (Table 1).| | Circle of Willis | Baseline | ||
| Variable | t-value | p-value | t-value | p-value |
| Age | 2.7 | 0.012 | -0.6 | 0.6 |
| Heart rate | -0.5 | 0.6 | 0.2 | 0.8 |
| Gender | -0.7 | 0.5 | -0.9 | 0.4 |
| Motion | -1.0 | 0.3 | 0.4 | 0.7 |
Discussion
In general, arterial blood flow consists of the steady and the pulsatile flow32-34. The former is affected by peripheral vascular resistance, the latter by arterial stiffness. After the age of 60, increases in blood pressure are mainly attributable to a rapid increase of pulse pressure (the difference between systolic and diastolic pressure), driven by the increase in systolic pressure35. We suggest that increased pulse pressure in cerebral arteries causes increased pulse amplitude in the COW, because the pulsatile blood flow increases relatively more with age than the steady flow. An alternative explanation for larger pulse wave amplitudes could be that the arterial compliance (the ratio of blood volume and blood pressure) increases with age in the circle of Willis. However, due to the lack of information about intracranial blood pressure, and since this would contradict the general trend of an increase of arterial stiffness with age, this seems to be less likely.It is not clear from our data whether the increase in waveform amplitude is due to a change of the wave form before it enters the brain, due to a change inside the cerebral arteries, or due to an altered wave reflection at the arteriole/capillary level. A more thorough investigation would have to involve the measurement of wave forms at the aortic or carotid level, and MRI of larger brain regions. It is neither clear from the present data if an actual re-shaping of the waves or an amplification, or a combined effect thereof, takes place. With the advent of even faster imaging methods such as multiband EPI24,36, studies like these are now within reach and subject of further research.
Acknowledgements
The authors acknowledge help from David Parker and Amirreza Sedaghat. Disclosure: The corresponding author’s institution filed a patent application related to the hypersampling method used in this manuscript.
References
1 Henry Feugeas, M. C. et al. Age-related cerebral white matter changes and pulse-wave encephalopathy: Observations with three-dimensional MRI. Magn. Reson. Imaging 23, 929-937 (2005).
2 Guyton, A. C. & Hall, J. E. Textbook of Medical Physiology. 11th edn (Saunders/Elsevier 2006).
3 Laurent, S. et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 27, 2588-2605 (2006).
4 O'Rourke, M. F. & Hashimoto, J. Mechanical factors in arterial aging: A clinical perspective. J Am Coll Cardiol 50, 1-13 (2007).
5 Tarumi, T. et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cerebr Blood F Met 34, 971-978 (2014).
6 Henskens, L. H. G. et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52, 1120-1126 (2008).
7 Tarumi, T., Shah, F., Tanaka, H. & Haley, A. P. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 24, 1108-1113 (2011).
8 Hughes, T. M. et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology 81, 1711-1718 (2013).
9 Tarumi, T. et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J. Cereb. Blood Flow Metab. 34, 971-978 (2014).
10 Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: A beginner's guide. Neurochem. Res. 40, 2583-2599 (2015).
11 Bacyinski, A., Xu, M. S., Wang, W. & Hu, J. N. The paravascular pathway for brain waste clearance: Current understanding, significance and controversy. Front. Neuroanat. 11 (2017).
12 Vrselja, Z., Brkic, H., Mrdenovic, S., Radic, R. & Curic, G. Function of circle of Willis. J Cerebr Blood F Met 34, 578-584 (2014).
13 Dagli, M. S., Ingeholm, J. E. & Haxby, J. V. Localization of cardiac-induced signal change in fMRI. NeuroImage 9, 407-415 (1999).
14 Chang, C., Cunningham, J. P. & Glover, G. H. Influence of heart rate on the BOLD signal: The cardiac response function. NeuroImage 44, 857-869 (2009).
15 Voss, H. U., Dyke, J. P., Ballon, D. J., Schiff, N. D. & Tabelow, K. Magnetic Resonance Advection Imaging (MRAI) depicts vascular anatomy. Conference abstract OHBM - Human Brain Mapping, 2436 (2015).
16 Voss, H. U., Dyke, J. P. & Ballon, D. J. Mapping cerebrovascular dynamics with MR advection imaging (MRAI): Estimation bias and image reconstruction challenges. Conference abstract ICERM - Computational and Analytical Aspects of Image Reconstruction (2015).
17 Voss, H. U., Dyke, J. P., Tabelow, K., Ballon, D. J. & Schiff, N. D. Magnetic resonance advection imaging (MRAI): Sensitivity to pulsatile flow. Conference abstract BRAIN/PET 2015, 141 (2015).
18 Voss, H. U., Dyke, J. P., Tabelow, K., Schiff, N. D. & Ballon, D. J. Mapping cerebrovascular dynamics with magnetic resonance advection imaging (MRAI): Modeling challenges and estimation bias. Conference abstract Meeting of the Society for Neuroscience (2015).
19 Bianciardi, M. et al. The pulsatility volume index: An indicator of cerebrovascular compliance based on fast magnetic resonance imaging of cardiac and respiratory pulsatility. Philos T R Soc A 374, 1-19 (2016).
20 Voss, H. U., Dyke, J. P., Tabelow, K., Schiff, N. D. & Ballon, D. J. Magnetic resonance advection imaging of cerebrovascular pulse dynamics. J. Cereb. Blood Flow Metab. 37, 1223-1235 (2017).
21 Hubmer, S., Neubauer, A., Ramlau, R. & Voss, H. U. On the parameter estimation problem of magnetic resonance advection imaging. Inverse Problems and Imaging 12, 175-204 (2018).
22 Voss, H. U., Stadler, J., Dyke, J. P. & Ballon, D. J. A transfer function model for local signal propagation in spatiotemporal MR data. Proceedings of the International Society for Magnetic Resonance in Medicine, 155 (2018).
23 Hubmer, S., Neubauer, A., Ramlau, R. & Voss, H. U. A conjugate-gradient approach to the parameter estimation problem of magnetic resonance advection imaging. Inverse Probl Sci En 28, 1154-1165 (2020).
24 Tong, Y. J., Hocke, L. M. & Frederick, B. D. Short repetition time multiband echo-planar imaging with simultaneous pulse recording allows dynamic imaging of the cardiac pulsation signal. Magn. Reson. Med. 72, 1268-1276 (2014).
25 Voss, H. U. Hypersampling of pseudo-periodic signals by analytic phase projection. Comput. Biol. Med. 98, 159-167 (2018).
26 Aslan, S., Hocke, L., Schwarz, N. & Frederick, B. Extraction of the cardiac waveform from simultaneous multislice fMRI data using slice sorted averaging and a deep learning reconstruction filter. NeuroImage 198, 303-316 (2019).
27 Voss, H. U. Intracranial pulse waves derived from dynamic MRI. Conference abstract Allen Workshop BioImage Informatics ( 2019).
28 Voss, H. U., Dyke, J. P., Ballon, D. J. & Gupta, A. MRI pulse wave profiles of cerebral arteries. Proceedings of the International Society for Magnetic Resonance in Medicine, 3247 ( 2019).
29 Jiang, H. X., Zhang, K., Wang, J. Y., Wang, X. Y. & Huang, P. F. Anomaly detection and identification in satellite telemetry data based on pseudo-period. Appl Sci-Basel 10 (2020).
30 Cohen, L. What is a multicomponent signal? ICASSP-92 -International Conference on Acoustics, Speech, and Signal Processing 1-5, E113-E116 (1992).
31 Sethares, W. A. Repetition and pseudo-periodicity. Tatra Mt. Math. Publ. 23, 1-16 (2001).
32 Fung, Y. C. Biomechanics: Circulation. 2nd edn (Springer 1997).
33 Zamir, M. The Physics of Pulsatile Flow. (AIP Press; Springer 2000).
34 Li, J. K. J. Dynamics of the Vascular System. (World Scientific 2004).
35 Franklin, S. S. Hypertension in older people: Part 1. J. Clin. Hypertens. (Greenwich) 8, 444-449 (2006).
36 Moeller, S. et al. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 63, 1144-1153 (2010).
Figures
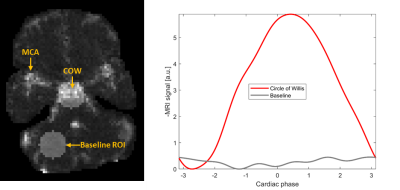
Figure 1: MRI pulse waves. Left: The MRI pulse wave amplitudes over the brain slice acquired with ultrafast MRI, for a single subject. The region of interest for the analysis of the circle of Willis and the baseline region without dominant pulsatility are highlighted. MCA = Middle cerebral artery. Right: The maximum-amplitude MRI pulse wave in the circle of Willis shows the characteristic systolic upstroke starting at around phase value -2.5 rad. The baseline signal is shown in gray.
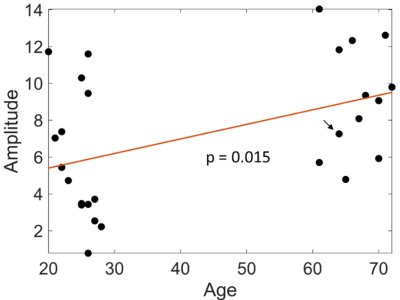
Figure 2: Increase of mean pulse amplitudes with age in the circle of Willis. The post-hoc regression line slope is significantly different from zero with a p-value of 0.015. The amplitudes used here were normalized by the median baseline amplitude for each subject. The subject of Figure 1 is marked with an arrow.